Power Shift - Big Battery Breakthrough
Air Date: Week of August 22, 2014
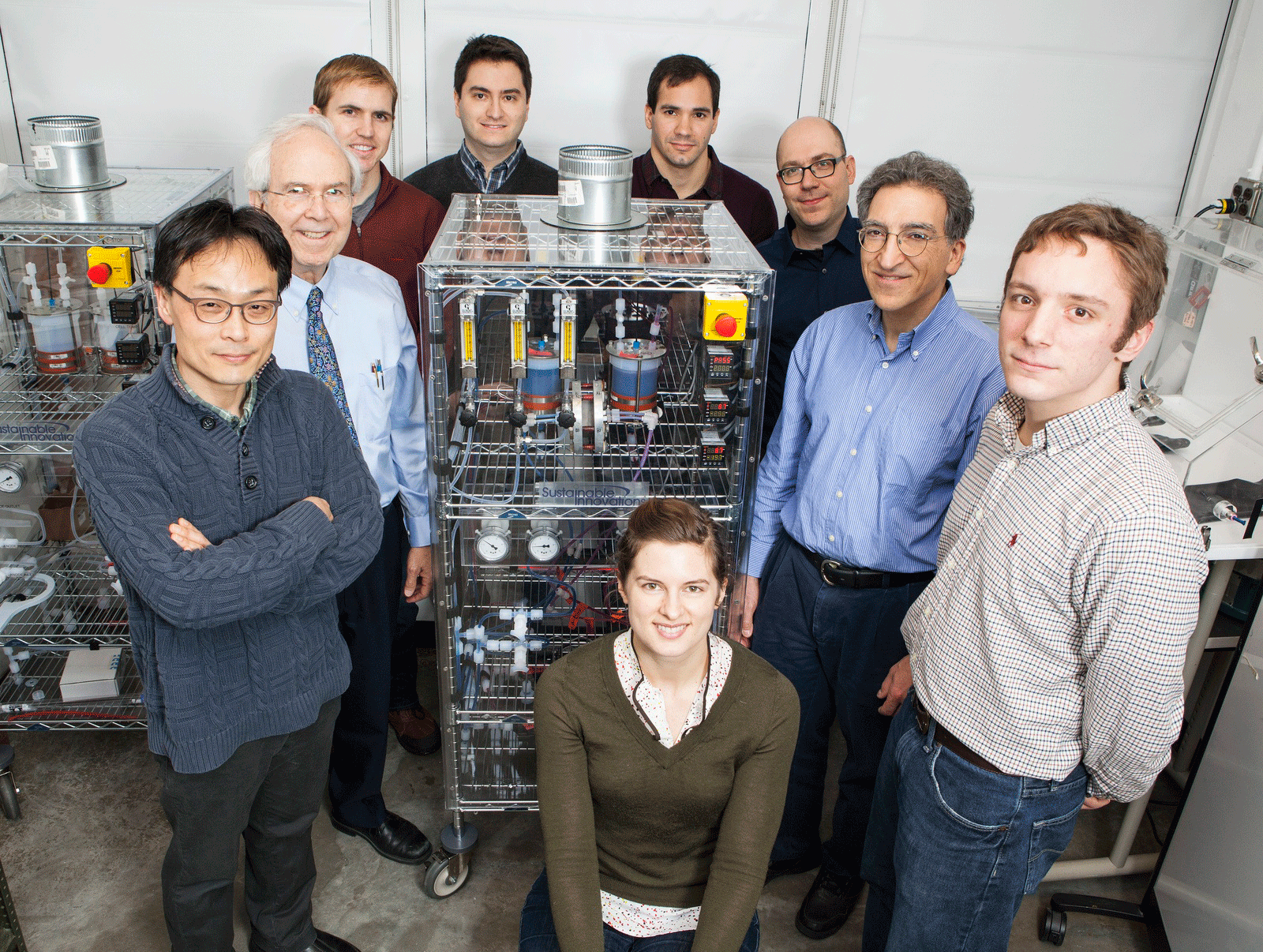
Harvard’s Flow-Battery Team clockwise from left: Dr. Changwon Suh, Prof. Roy G. Gordon, Dr. Brian Huskinson, Dr. Suleyman Er, Dr. Michael Marshak, Prof. Alán Aspuru-Guzik, Prof. Michael J. Aziz, Michael Gerhardt, and Lauren Hartle. (Photo: Eliza Grinnell, Harvard School of Engineering and Applied Sciences)
A breakthrough in battery technology could make large-scale energy storage available for renewables both on and off the grid. Researchers at Harvard University say their organic flow-battery would be affordable and efficient. Professor Michael Aziz tells host Steve Curwood how the technology works and why it could significantly improve the distribution of wind and solar power.
Transcript
CURWOOD: Well, maybe concern about the looming climate crisis has you planning to go solar at your home or business. But even if you do, come the evening you have to buy electricity or use very expensive batteries to keep the lights on. Utilities with large scale solar and wind farms also have to manage these intermittent power sources. And much of power from the always-on nuclear and coal plants goes to waste at night because demand is low and it’s too expensive to store the energy. But some Harvard researchers have made a major breakthrough in developing cheap batteries that could store massive amounts of electricity. Michael Aziz of Harvard’s School of Engineering and Applied Sciences is one of the project leaders and he came into the studio - Professor Aziz, welcome to Living on Earth!
AZIZ: Thank you. It’s a pleasure to be here.
CURWOOD: Now, your team has created what’s called this organic megaflow battery, but before you tell us what that means, first of all, just outline what are the problems for conventional batteries for renewable energy sources.
AZIZ: Sure. The two most important measures of the capability of a battery are the amount of energy in can store, and that’s measured in kilowatt-hours. That’s what you pay for in your electric bill, how much energy you’re using. And the rate at which you can deliver that energy out of storage, and that’s the power measured in kilowatts. The ratio of energy to power is very different for different applications, and traditional batteries are very limited in the range of that ratio they can come up with. So, for example, have you ever had a car that wouldn’t start, and you cranked and cranked the starter until the battery died?
CURWOOD: Ummm...it’s that time of year.
AZIZ: Well, the battery lasts maybe ten minutes, 15 minutes, until it’s drained. That ratio of energy to power is the number of hours you can discharge your battery at full power until it’s drained. It turns out for storing wind and solar, you need many hours to days, and there’s no traditional battery that can do that inexpensively.
CURWOOD: In other words, the fundamental problem here, is that you need batteries that can last for a long, long time, and today’s batteries aren’t designed to do that.
AZIZ: That’s right.
CURWOOD: So, your team has created what’s called organic megaflow batteries. What exactly does that mean? Organic...is that good for you to eat?
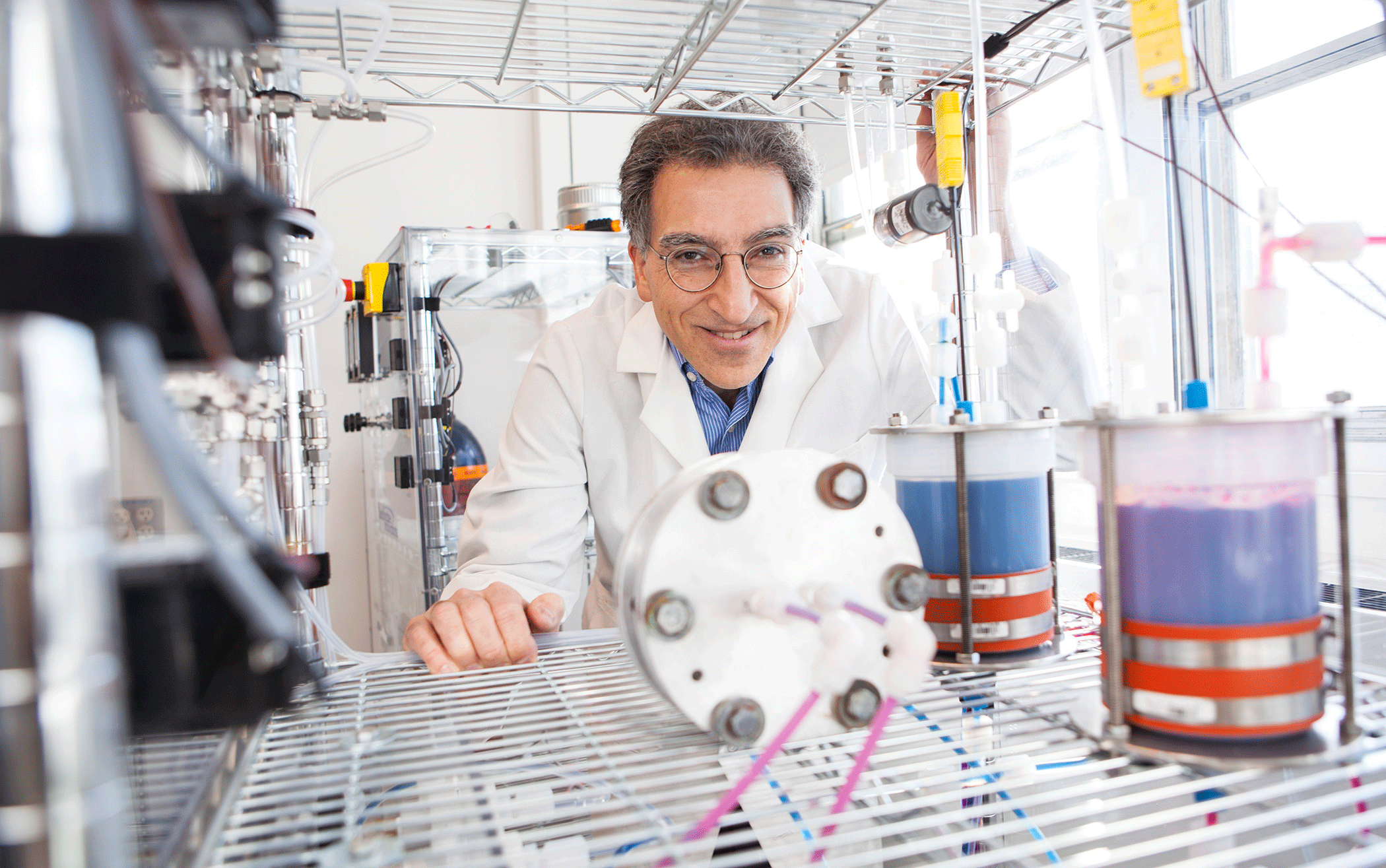
Professor Michael Aziz in his organic flow-battery laboratory. (Photo: Eliza Grinnell, Harvard School of Engineering and Applied Sciences)
AZIZ: Turns out that the molecules we’re using are very, very close to a molecule found in rhubarb. The chemicals are in your green vegetables...you eat them every day. The flow battery is a different concept.
CURWOOD: What is a flow battery?
AZIZ: The flow battery is different from a traditional solid electrode battery because it stores the energy outside the battery container itself in storage tanks full of fluids. It’s a lot like a fuel cell. In a fuel cell you store the energy outside the fuel cell itself, for a fuel cell car it would be hydrogen and air. When you want electricity, you flow those chemicals into the cell past the electrodes where they’re converted to low energy chemicals and they give off the energy in the form of electricity, and you exhaust the low energy product. But for a flow battery, you can’t exhaust the product, you’ve got to contain them so when it’s time to charge up the battery, you flow those product chemicals back into the cell, drive in electricity and convert those chemicals back to the original high energy chemicals that you started with. Now you have a battery.
CURWOOD: So a flow battery...it could be any size that you want. If you store the energy in a tank separate from where the electrodes come together, the bigger the tank, the more energy you could store.
AZIZ: That’s exactly right. You can design the electrodes to give you the power you need, and then you can get arbitrarily large amounts of energy by just bigger and bigger tanks full of chemicals, and that’s potentially a much cheaper way of storing large amounts of energy than stacking up entire banks of solid electrode batteries.
CURWOOD: So there are a few flow batteries already operating out there, large-scale electric plants as I understand it. How correct is that?
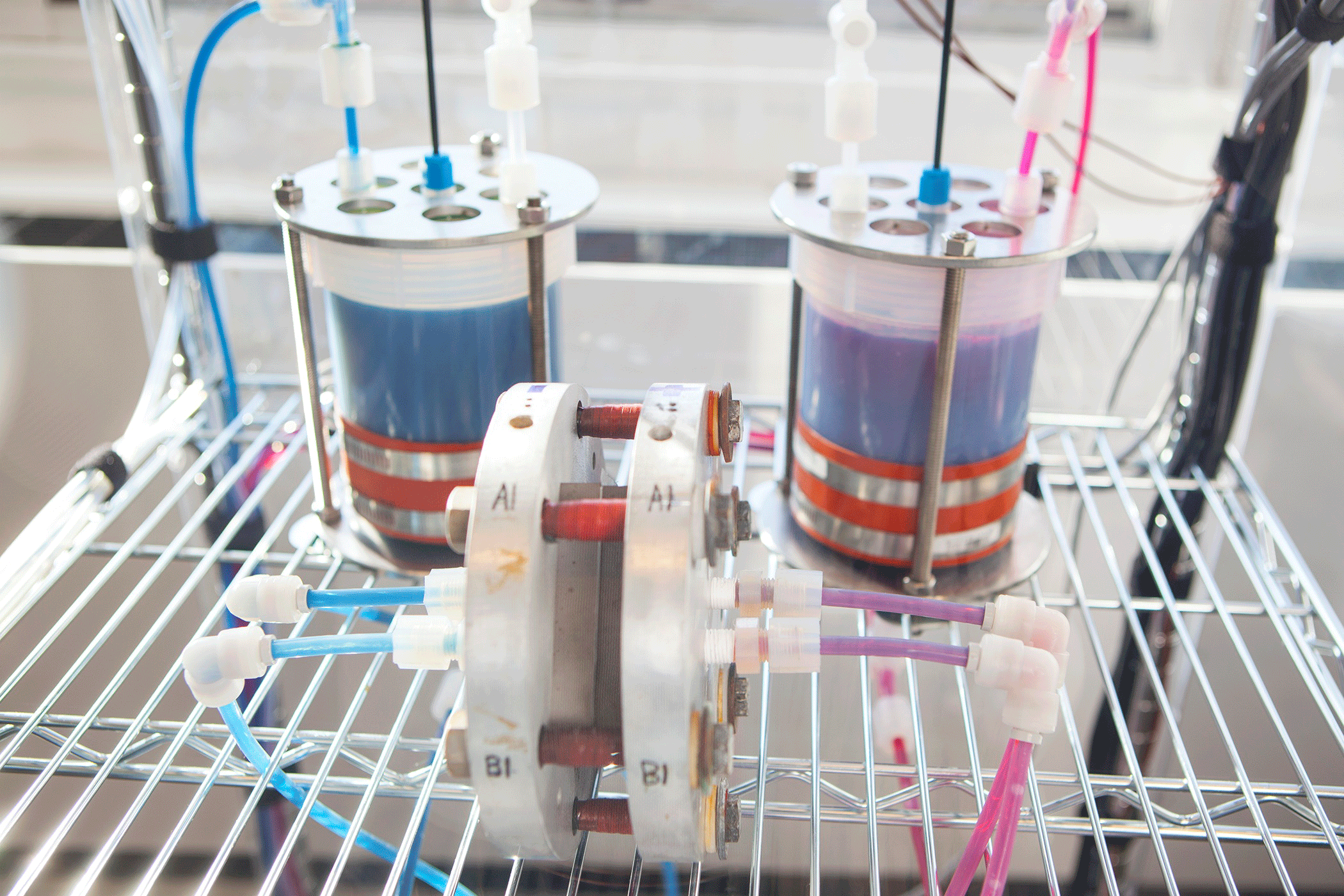
Organic mega-flow battery in the Harvard SEAS lab. (Photo: Eliza Grinnell, Harvard School of Engineering and Applied Sciences)
AZIZ: That’s right. There’s several different flow battery chemistries that are being developed. The most commercially advanced stores energy in the form of Vanadium ions in an aqueous solution, and the problem with that is Vanadium is a rare and expensive metal.
CURWOOD: But you don’t use Vanadium. You use something that, well, you find in food.
AZIZ: As a matter of fact, I noticed that other groups were making progress on designing fuel cells using organics, and so we started looking around for organic molecules that might work well in a flow battery. We noticed this class of molecules in chlorophyll called quinones. When they’re in chlorophyll during photosynthesis, they switch back and forth between oxidized and reduced forms over and over again without any sign of degradation, and that’s exactly the functionality we want in a battery. So we modified them to make them highly soluble in water, and put them in a flow battery, and it works. Performance is terrific. After half a year at this, the performance is rivaling that of Vanadium. We haven’t had a chance to optimize anything yet, so we think that we have a lot of room for improvement ahead of us.
CURWOOD: So, part of your secret sauce is that you’re imitating nature.
AZIZ: That’s right. We find that nature has learned how to do things really well, and in this case going between oxidized and reduced states over and over again without degrading is something nature figured out how to do in order to make photosynthesis work so we’ve adopted that now in a battery.
CURWOOD: So these quinones are really abundant...you can find it in rhubarb, huh?
AZIZ: There’s a quinone in rhubarb that is so, so close to the molecules that we actually have in our first quinone flow battery that some people are calling it a rhubarb battery. Right now what we have comes from crude oil because it’s very cheap and the most important thing is to get storage to be cheap or else it can’t be useful. Ultimately, if we can get it out of rhubarb that would be an extra bonus.
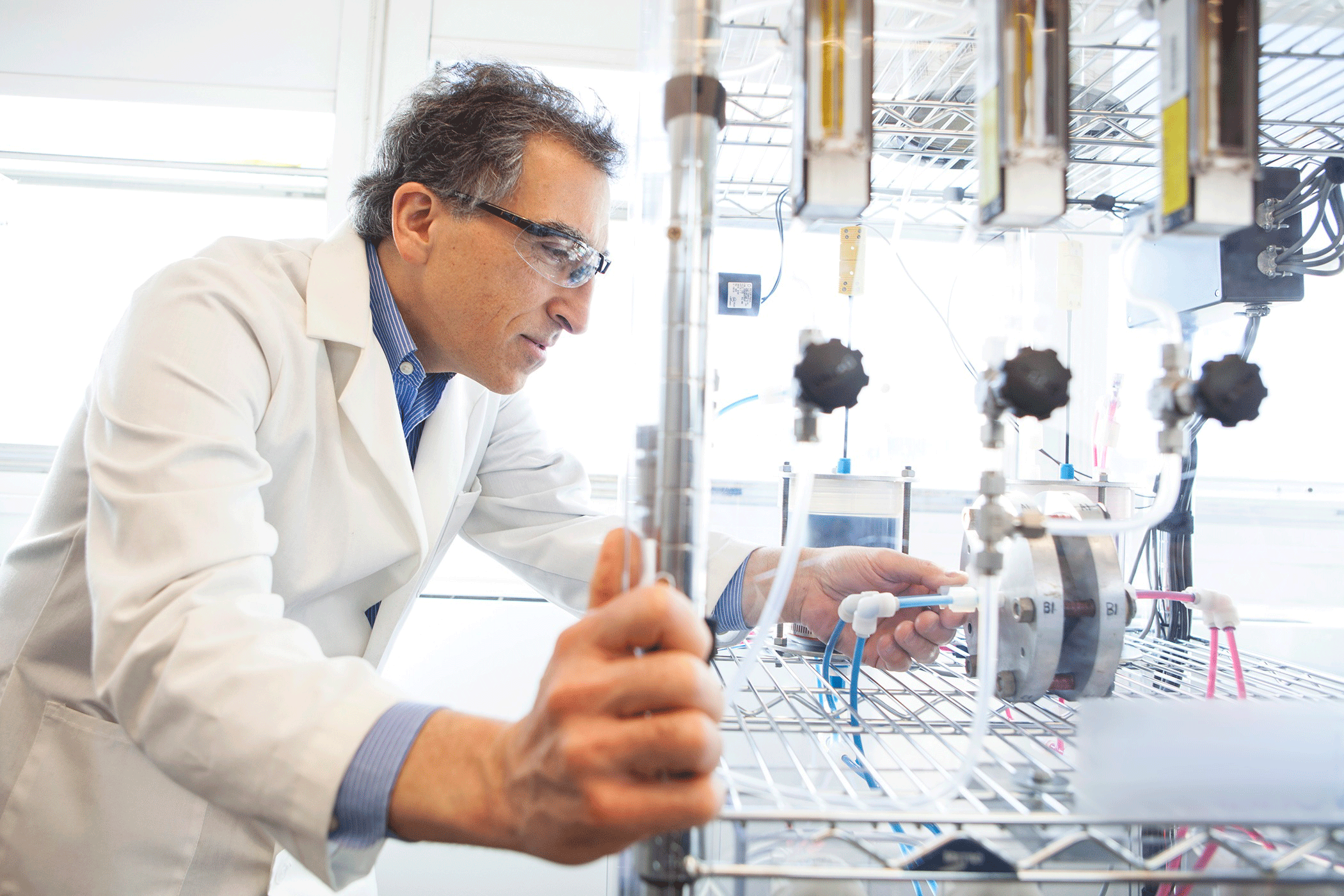
Professor Michael Aziz working in Harvard’s SEAS lab. (Photo: Eliza Grinnell, Harvard School of Engineering and Applied Sciences)
CURWOOD: What would this look like, say, installed in my basement to support home solar cells?
AZIZ: It would be a power conversion unit that would look like an electrical machine, and it would be hooked up to two storage tanks, one for the positive electrolyte and one for the negative electrolyte. Separating the positive and negative that way is a safety feature of flow batteries that you can’t get in solid electrode batteries like lithium ion. There, the positive and negative are all mixed together in the same cell.
CURWOOD: So the two tanks makes it safer?
AZIZ: The two tanks make it safer because the chemicals can’t all mix together at once and give you a catastrophic discharge like you can get in lithium ion batteries for example.
CURWOOD: And these two tanks would be no bigger than, say, a typical oil tank or something?
AZIZ: That’s right. For a typical set of solar panels on your rooftop, you’d have two tanks, the total volume of which would be the size of one home heating oil storage tank in the basement.
CURWOOD: To what extent do you feel your development could really revolutionize our use of solar and wind power?
AZIZ: The way I see it, the biggest problem in us getting most of our electricity from the wind and the sun is their intermittency. We need a battery that can safely and cost-effectively store large amounts of electric energy, and this has a fighting change of being able to do that.
CURWOOD: Michael Aziz is Professor of Materials and Energy Technologies at Harvard’s School of Engineering and Applied Sciences. Thanks so much for coming by.
AZIZ: My pleasure.
Links
Nature Magazine research paper on Harvard’s flow battery
Living on Earth wants to hear from you!
Living on Earth
62 Calef Highway, Suite 212
Lee, NH 03861
Telephone: 617-287-4121
E-mail: comments@loe.org
Newsletter [Click here]
Donate to Living on Earth!
Living on Earth is an independent media program and relies entirely on contributions from listeners and institutions supporting public service. Please donate now to preserve an independent environmental voice.
NewsletterLiving on Earth offers a weekly delivery of the show's rundown to your mailbox. Sign up for our newsletter today!
 Sailors For The Sea: Be the change you want to sea.
Sailors For The Sea: Be the change you want to sea.
 The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
 Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
 Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth
Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth