A Novel Way to Capture and Release the Warmth of the Sun
Air Date: Week of February 5, 2016
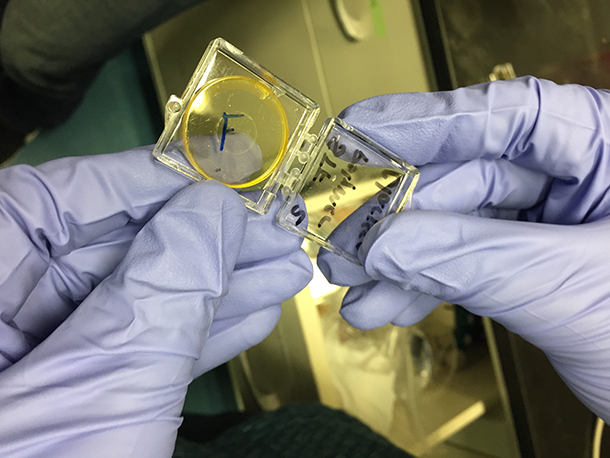
The solar heat-storing material is a thin, yellowish-orange film. (Photo: Steve Curwood)
Storing solar energy is an enduring challenge for scientists, but now a team of MIT researchers has developed a new material that can trap it and release it as heat on demand. Host Steve Curwood visits the MIT lab to hear from postdoc David Zhitomirsky and graduate student Eugene Cho about their material and how it might be used to do such things as defrost windshields and warm our clothes.
Transcript
CURWOOD: It’s Living on Earth, I’m Steve Curwood. If you drive in the colder climes, on many mornings you need to scrape your windshield clear of ice. But you can de-ice the back window with just the push of a button. That’s because it's illegal to put anything in windshield glass that could block the driver’s view, such as heating wires. But now a team of researchers at MIT has come up with a chemical that would let windshield glass directly store solar energy and then release it on demand as heat to melt the ice.
This chemical stores and releases energy by changing shape and could be very useful in electric cars that don’t have engine heat for defrosting. And the same chemical could be woven into clothing fibers to capture the sun’s energy and then give you some added warmth when you ask for it, even days later. I paid a visit to the lab where the MIT team has been working on this breakthrough and met up with researchers David Zhitomirsky and Eugene Cho, who work in the lab of professor Jeffrey Grossman.
[SOUNDS OF THE LAB]
Hi, David. Hi, Eugene.
ZHITOMIRSKY: Thanks.
CHO: Hello.

In the early stages of research, the solar heat material proved difficult to deposit as a uniform film and crumbled away at the edges. (Photo: Steve Curwood)
CURWOOD: Let's go to the basics here, David. Now, most people, when they think of collecting solar energy, they think of converting it to electricity, but this is a chemical process. Tell me what you do.
ZHITOMIRSKY: So yes, it is quite different from solar cells where you absorb light and convert it to an electrical charge. In this case, what we do is we use these molecules that can absorb UV light and instead of generating charges, what they do is that they change shape, and by changing shape, they can store chemical energy this way.
CURWOOD: OK, so sunlight hits this molecule, it changes shape and can storage its energy. And how do you get the energy out?
ZHITOMIRSKY: So you can figure the material in several ways. One way is to add a small amount of heat, and the material will release more heat than you add in. The other methods are triggering it with light or you can apply an electrical field to the material.
CURWOOD: What made you think that it would be possible to do this?
ZHITOMIRSKY: The field of these kind of fuels is not new. What is novel here is their obligation in solid-state that enables them to be utilized in consumer goods. And so we chose a molecule that was already known to be stable and that had this kind of behavior, but the key was to employ it in solid state and make very nice and uniform films.
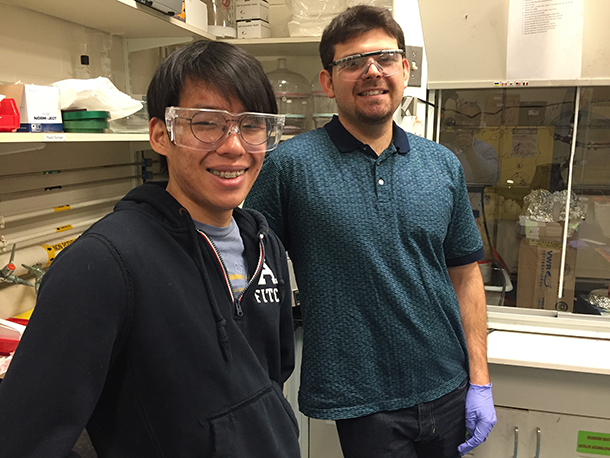
From left to right: graduate student Eugene Cho and postdoc David Zhitomirsky in the MIT lab. (Photo: Steve Curwood)
CURWOOD: OK. Introduce me to your molecule please, and let me get my mouth ready for the pronouncer.
ZHITOMIRSKY: So the molecule is called azobenzene.
CURWOOD: Azobenzene. That's not so hard to say.
ZHITOMIRSKY: Yep. And basically we take this azobenzene and we will polymerize it, meaning we link up units of azobenzenes together and that enables us to make very nice films.
CURWOOD: So if you can make a film, how transparent might this film be?
ZHITOMIRSKY: Right now it has a yellowish orange tinge, but in principle we have simulations that show that you can actually tailor the film to be fully transparent, and invisible.
CURWOOD: So, what were the big challenges of getting this to work? You understood the scientific principles here, but you we're looking to cut the costs and make it simple.
ZHITOMIRSKY: Yes, exactly. So, one of the directions that things moved in before I joined this group was putting these molecules on carbon nanotubes in order to improve their properties. And so that's expensive inherently because you need carbon nanotubes, and the solubility of the material wasn't very high. I come from a background in nanocrystals and we were using these nanocrystals back in Canada, Toronto, to absorb photons and convert them to electricity, so it was basically solar cell technology, and the idea there was to make a paint, a solar paint that you would simply put on a substrate and it would make everything much more inexpensive. This polymer that we have right now is also essentially a paint. You can deposit it directly from solution. The interesting thing for me was to expand the horizons of what you can do with energy instead of just converting light to electricity, now you can convert it to chemical energy and then ultimately release this energy as heat.

The team often works out chemical reactions on fume hood glass in the lab. (Photo: Steve Curwood)
CURWOOD: Whose idea was this?
ZHITOMIRSKY: It was a collective meeting between my supervisor and I and Eugene, and we just sort of sat there and we thought how could we make something that people were actually going to care about and this came out of it.
CURWOOD: Eugene Cho, you're a graduate student at MIT. Gene, why are you excited to work on this project?
CHO: It was a lot different from other research before because before when I joined back in 2012 what was big was solar panels. But everybody was working on that. I wanted to do something slightly different from the conventional solar panel industry and when I talked to Jeff he suggested this project where there's molecules, not large panels, that could absorb the sunlight and reuse it in different applications, in this case in the form of heat.
CURWOOD: So here we are in your laboratory at MIT. Give us a tour. Show us the machines and the material here. What's what?
ZHITOMIRSKY: So this is the fume hood, and we work in it because of toxic materials.
CURWOOD: How toxic is this stuff?
ZHITOMIRSKY: In small molecule form it's somewhat toxic, but as soon as you make a polymer out of it and that's another advantage - it suddenly becomes not so toxic at all. So in here we have things like pumps in order to evacuate containers of solvents because we need to dry things off. We have scales to weigh things out. We have all sorts of solvents in order to separate things out. We have some vacuum lines and nitrogen lines. This is called the Schlenk line.
CURWOOD: And what you have scribbled here on the window?
ZHITOMIRSKY: Sometimes we just write chemical equations and how we engineer these materials directly onto the glass.

A vacuum hood allows MIT researchers to safely work with toxic materials needed to make this film. (Photo: Steve Curwood)
CURWOOD: [LAUGHS] So what we have here?
[SOUNDS OF OPENING DRAWER]
ZHITOMIRSKY: So, here we have a film right on top of a glass substrate as you can see. It has an orange or yellow tinge, and this is actually an example of what a type of film we'd make to do also all sorts of investigations for heat release.
CURWOOD: Well, that's pretty clear. There's a little bit of yellow-orange around the edge there, but if you look through it it's...a pair of sunglasses would cut out more light like that. Now, I understand one of the applications for this might be in the windshield of the car, to de-ice a car. How would that work?
ZHITOMIRSKY: What you would do is you put this material inside of the windshield and when you wanted to de-ice the windshield you would hit a button and it would trigger the material to release any energy that it has stored. And the idea is to melt the layer of ice right between main layer of ice and the windshield itself. So you're not actually melting all the ice, just a thin sheet of it, and you can remove it with a windshield wiper for example.
CURWOOD: It would just slide right off. So what's your best guess as to how you get from the yellow, the yellowish orange, to something that's really completely transparent, translucent?
ZHITOMIRSKY: The reason this molecule results in a yellow film is because it absorbs along parts of the visible spectrum, and what you can do is you can attach different types of functional groups onto the molecule and this will shift the absorption spectra around.
CURWOOD: People are very interested in this, I gather, because in electric cars you use a lot of energy to do something like de-ice or heat the car.
ZHITOMIRSKY: Exactly, yes, now instead of running electricity through metal wires or the metal screen, you simply employed energy that you get for free just by absorbing sunlight.
CURWOOD: By the way how much energy is used in an electric car for things like de-icing the windshield or heating the people?
ZHITOMIRSKY: We've estimated approximately 30 percent, so this kind of technology will certainly be able to make a dramatic impact on electric vehicles.
CURWOOD: Now, one thing that I'm really intrigued about is the notion of heating clothes. So if I walk outside on a sunny day and my clothing get some energy in it and then later when it's colder I could just hit a button and it'll keep me warm like an electric blanket?
ZHITOMIRSKY: So, this is one of the applications that is closer to the market because you can feel as much as two degrees or three or maybe five degrees, and this is achievable today with these materials. The way we envision using it is to integrate into fibers that you then make clothing out of, or what you can do is just take a separate sheet of the stuff, and in certain cases it might be appropriate to just attach a sheet of the stuff onto the clothing itself.
CURWOOD: So this could be like an updated blanket or sheet. Hang the sheet outside, it gathers all this energy and have it at night and then if it's a little cold, hit a switch and I've got something a little bit warmer.
ZHITOMIRSKY: Yes, exactly, you could use it as a solar blanket, for sure, and it might be much more appropriate for places that don't have an electrical outlet, for example, if you're camping or especially in third world countries where they don't necessarily always have access to electricity, this would be quite useful to staying warm at night.
CURWOOD: So, there I am on my expedition with my solar blanket. What would release the heat?
ZHITOMIRSKY: So you have to have a system that can trigger this material and a simple one might be carrying around a blue LED with you that could just get this process going.
CURWOOD: So you zap it with more light and it says oooh, I'm free now.

Host Steve Curwood, left, in the MIT lab with Eugene Cho, center, and David Zhitomirsky, right. (Photo: Helen Palmer)
ZHITOMIRSKY: Exactly, use a different wavelength of light and you could trigger it that way.
CURWOOD: That could be a problem. You're walking down the street and someone happens to have one of those things, and ohhh, I'm feeling a little warm now.
ZHITOMIRSKY: Yeah, that's certainly one thing to get around, making sure that this material is only exposed to the right kind of radiation at the appropriate time.
CURWOOD: OK, this sounds great in theory, but is it affordable to put it in something like clothes or a windshield?
ZHITOMIRSKY: So that process itself is very simple. It takes two synthesis steps to make the material itself and you can make it in a on a very large-scale, on kilogram scale. We've easily scaled our stuff up to grams, and as far as the cost of the actual material, it's actually quite low.
CURWOOD: Now, you are a graduate student, Eugene, so what's your thesis going to be about?
CHO: Hopefully to get these molecules to work!
CURWOOD: [LAUGHS] Let's go forward 10 or 20 years. How do you think this work will have been applied in the coming years?
CHO: Well, I guess with this it could expand into, with high-energy density store, be a stovetop or used to de-ice like pipes types in bigger scales rather than just windshields. Larger applications I guess.
CURWOOD: So how long before we see this coming to market?
ZHITOMIRSKY: I would say that it is possible somebody will pick this up as a startup fairly soon, maybe in a length's scale of a year or two because there's things you can do with these materials right now.
CURWOOD: David Zhitomirsky is a Banting Fellow post-doc associate. Eugene Cho is a master's student at the Massachusetts Institute of Technology working on solar thermal fuels with MIT professor Jeff Grossman. Gentleman, thanks so much for taking the time to show us around your lab.
ZHITOMIRSKY: Thank you very much.
CHO: Thank you.
Links
Journal article, “Solid-State Solar Thermal Fuels for Heat Release Applications”
Living on Earth wants to hear from you!
Living on Earth
62 Calef Highway, Suite 212
Lee, NH 03861
Telephone: 617-287-4121
E-mail: comments@loe.org
Newsletter [Click here]
Donate to Living on Earth!
Living on Earth is an independent media program and relies entirely on contributions from listeners and institutions supporting public service. Please donate now to preserve an independent environmental voice.
NewsletterLiving on Earth offers a weekly delivery of the show's rundown to your mailbox. Sign up for our newsletter today!
 Sailors For The Sea: Be the change you want to sea.
Sailors For The Sea: Be the change you want to sea.
 The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
 Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
 Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth
Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth