Bird Flu Warning
Air Date: Week of January 31, 2025

Avian flu is rampaging through poultry farms, driving up egg prices. (Photo: Alexxx Malev, Wikimedia Commons, CC BY-SA 3.0)
So far avian flu hasn’t been seen spreading from human to human, but recent mutations indicate some variants are becoming better adapted to infecting humans. Dr. Richard Webby of St. Jude Children’s Research Hospital also directs a World Health Organization center on the ecology of influenza. He joins Host Aynsley O’Neill to explain what we know about bird flu so far, and how we can prepare for the possibility of a pandemic.
Transcript
DOERING: From PRX and the Jennifer and Ted Stanley Studios at the University of Massachusetts, Boston this is Living on Earth. I’m Jenni Doering.
O’NEILL: And I’m Aynsley O’Neill.
The cold winter months are typically thought of as cold and flu season, and in recent years, we've also heard a lot about COVID19 and RSV around this time. But there’s another virus now making headlines: H5N1. That's the name for a variant of influenza we know as bird flu, and the virus itself has been around for decades. But here at the start of 2025, it has been running rampant in the wild bird population, it’s destroying flocks of chickens, and has mutated to infect cows. One variant even resulted in a human death in Louisiana in recent weeks and hospitalized a Canadian last fall. For more, we turn now to Dr. Richard Webby of St. Jude Children’s Research Hospital.
He’s also the director of the Collaborating Centre for Studies on the Ecology of Influenza in Animals and Birds at the World Health Organization. Dr. Webby, welcome to Living on Earth!
WEBBY: Well, thanks Aynsley. It's great to be here.
O'NEILL: So what is the distinction between H5N1 and your typical seasonal flu?
WEBBY: The major difference between these are the hosts that they're associated with. So the seasonal flu that we get every winter, that's a virus that is maintained just within humans. So its goes human to human to human. There's no involvement of any other animal species in the maintenance of that virus. What is the ancestry of those viruses? So at one stage, they were avian viruses. All influenza viruses exist as the natural host in the aquatic bird reservoirs of the world. So the human seasonal strains went from birds to humans, now maintained in humans. The H5, again, so genetically, it's related to the viruses in humans, they're all influenza viruses, but it's a virus that is much more adapted for replication still in the avian host, it hasn't adapted to humans. So I guess the real answer to your question is, they're all influenza viruses, but they have slight differences in terms of how they interact with host cells, how they replicate. One prefers to bind to avian cells, that's the H5. The others prefer to bind to human cells.

The H5N1 virus, also known as bird flu, has a variant that has mutated to infect dairy cows. Experts warn that unpasteurized milk could be contaminated and dairy workers could become infected. (Photo: RF Vila, Wikimedia Commons, CC BY-SA 4.0)
O'NEILL: And from what I have heard, the very serious cases and the fatality are likely caused by the outbreak in wild birds, as opposed to being traced to maybe the bovine outbreak. Correct?
WEBBY: That's correct. I think, if you're sort of summing up where we are in the US now with the H5 situation, we've already got two fronts going on. We have the virus -- again, went from birds into cows in early 2024, maybe late 2023 -- that's maintained primarily just in cows now, with the odd spill over to other hosts. And then the other front is the virus that caused the more severe infections in Canada and Louisiana. It has also caused mild infections as well. We can't forget about that, but that is a purely a wild bird driven event. So as the migratory birds came down from up north, they brought this particular version of the virus with them. So, H5 in both wild birds, poultry and cows, but you can think of it probably like the variants with COVID: slight variants of the same virus in each of those hosts right now.
O’NEILL: If the virus mutates to become transmissible, human to human, what do you think is likely to happen? You know, should we be gearing up for the next COVID-like pandemic?
WEBBY: [SIGHS] Oh, I really wish I had the answer to this one, Aynsley. It's tough, right? So intrinsically, this H5N1 virus, at least in my mind, has the capacity to cause far more severe disease than the Coronavirus that caused the COVID pandemic. At least what we're seeing in terms of many of the hosts that are being infected with it. We're not seeing that same degree of disease in most of the humans to date, but that's probably because this virus, as you sort of alluded to, is not well adapted to humans right now. So should it change, we don't really have a good idea of what disease may look like, but on paper, we've been looking at influenza viruses for many decades here at St. Jude, and this is the nastiest virus that we have dealt with. So the potential is really, really scary, but bottom line is, we don't know. Would we be faced with worst case scenario, middle case scenario, or best case scenario? Unfortunately, it's something we just don't, we don't have the answer to, so we're just got to, what we got to do is be prepared for the worst case scenario and hope that's not the case.

Dr. Richard Webby is a member of St. Jude Children’s Research Hospital’s faculty and the director of the World Health Organization’s Collaborating Centre for Studies on the Ecology of Influenza in Animals and Birds. (Photo: Courtesy of St. Jude Children’s Research Hospital)
O'NEILL: Now, what about vaccines? What is the status of any vaccinations that may be in progress, and how would they possibly affect the reach of this virus?
WEBBY: There's sort of multi fronts to that question. One, we can vaccinate cows. Two, we can vaccinate chickens. Or three, we can vaccinate humans. If we look at those sort of individually, from the cow front, there are vaccines that are being developed and are being tested. Exactly where they are in the process, I'm not sure, but you know, certainly field testing was initiated on those vaccines. So theoretically, we could have a vaccine to immunize cows, should that be needed. From a poultry front, there are approved, licensed poultry vaccines that are used across the globe. They're not approved for use in the US because of trade barriers that come about through use of vaccines in birds. So they're there if they're needed, but they're not approved for use. In humans, if we think of all the nasties that are circulating in animal populations that human health is worried about, we're probably more prepared for an H5 in terms of vaccines than anything else. Studies have been going on for two decades with H5 vaccines in humans -- how do we formulate them best? How do we best deliver them? What's the best way to grow them and make them? And so from that perspective, we know how to make an H5 vaccine, unlike the beginning of the COVID pandemic, where we really had no idea. So we know how to make them. We've made them. We've tested them. Some countries, including the US, have some amounts of vaccine stockpiled, which could be useful at the very, very beginning. And of course, the US and other countries have also invested in some of these newer technologies as well. So the vaccines I talked about, there are more of the traditional shot-in-the-arm vaccines that we're used to. The mRNA technologies, of course, they have the capacity to contribute to a response as well. And the US government has invested heavily in pushing some of the mRNA vaccines forward as well.

Dr. Webby warns duck hunters to be careful when handling their catches because they could be infected with H5N1. (Photo: Alain Carpentier, Wikimedia Commons, CC BY 3.0)
O'NEILL: Yeah, I remember mRNA vaccines, I mostly associate with the COVID 19 pandemic. What have you heard about that development? And to what extent are you hopeful about it or not so hopeful?
WEBBY: Yeah, so the mRNA vaccines, a lot of them were being tested on flu before the COVID pandemic came along. The US government has invested heavily in this to bring this technology along as a backup to traditional methods, should a pandemic occur. Where are those vaccines now? They're being made. They're being tested. The manufacturers can make the vaccines fine. What we don't really know is how well they work in humans against the H5 virus. So H5 vaccines historically have been a little bit poor in their ability to induce a really robust immune response. So while I absolutely think we need to invest and find out how an mRNA vaccine would work, we don't yet know how that will behave in humans when targeting an H5 virus.
O'NEILL: What can individuals be doing about this to reduce their risk?
WEBBY: I think it's important to say, yeah, if you're not in contact with dairy cattle or large poultry operations, your risk right now is exceedingly low. All the human cases have been bird or cow to human and no human to human transmission. So if you don't have those exposures, your risk is low. But what you can do, again, considering we have seen an uptick of activity of H5 virus in wild birds over the past few weeks, if you're out and about and you see a bird acting weirdly, meaning it's just walking in circles and looking very sick. Just give it a wide berth, and treat it like it could be potentially infectious. The other group that I quite often get asked questions about is duck hunters. Should they be worried? Just be a little bit sense about this. Just treat a bird like yes, potentially is infected. Chances of getting infected from it are slim, but it's not zero. So again, particularly if you're eviscerating the bird, cleaning the bird, just be really careful. Wear gloves, do it in an outside, ventilated area. But otherwise, there's not too much the average person needs to do or can be doing.
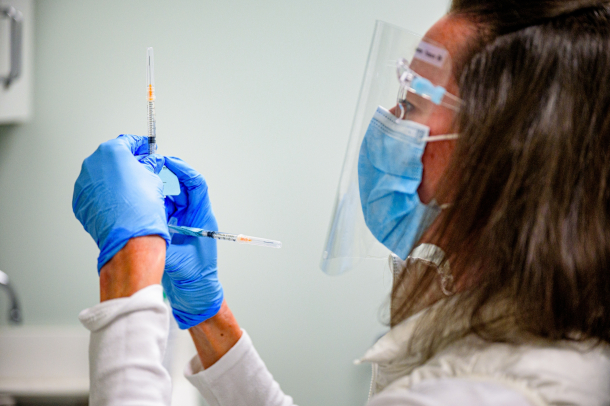
While a H5N1 pandemic could be catastrophic, unlike with the COVID vaccine shown here, scientists already have H5N1 vaccines stockpiled. They are likely, but not guaranteed, to be effective. (Photo: City of Greenville, North Carolina, Flickr, public domain)
O'NEILL: And then what about on a public health level?
WEBBY: So from a public health level, the three, I think, pillars, to the response are testing, ability to treat, and sort of a vaccine response. And you know, there have been efforts in all of those three pillars over the past few months. Resources are being put into increasing capacity to test for H5, increasing the availability of some of the flu antivirals, and also development of the vaccines as well. So from public health, there is some preparedness going on. Is it enough? No, it's never enough. But it does take resources, and it's resources for something that may never happen. So it's a challenging equation to get right.
O'NEILL: Dr. Webby, at this point, just how worried are you?
WEBBY: I tell people I'm a little more uneasy now than I was six weeks ago. So where were we six weeks ago? The big risk was infected dairy cattle, but the virus had been circulating in that host for now close to a year, and we haven't really seen that virus evolve into something that's a little bit more infectious for humans. We know, when we sequence the viruses from cows, we know some of the markers that we're looking for to suggest, okay, this is a virus. That's a bird virus, but these are some changes, mutations it’s making that probably make it more infectious for humans. We really hadn't seen much of that going on. But we are, what's happened more recently, one is the two severe cases in Canada and Louisiana. It wasn't so much the severity of infection that worries me, but when the Canadian authorities and the CDC sequenced the virus from those individuals, there was evidence that the virus was starting to make some of the key changes that would make it more infectious for humans, so starting to adapt to the human host. So before that, we hadn't seen any of the evidence the virus was making these changes. Now we've seen it so we know it probably can make those changes. And there's also a study that came out recently from investigators at the Scripps Institute in California, where they could show that a single mutation in the virus switched it from recognizing the preferred receptor for the avian virus to the preferred receptor for the human virus. And what does that mean? It just simply means that a single amino acid mutation could potentially make the virus bind to human cells better. It's a virus that's got to bind to the human cells to get inside and replicate. So combined, those two things are making me a little a little more uneasy. I don't think this virus is, meaning the H5, virus is a human pathogen. Certainly it's not a human pathogen yet, and I do think the barriers for it to overcome are probably pretty high. But the potential consequences of an H5 pandemic, if the worst case scenario were to occur, is frightening. So it, it’s, how to balance that? Yes, it's probably a rare event. But should that rare event occur, it's going to be awful.
O'NEILL: Dr. Richard Webby is a researcher at St Jude Children's Research Hospital and the director of the World Health Organization's Collaborating Center for Studies on the Ecology of Influenza in Animals and Birds. Dr. Webby, thank you so much for taking the time with me today.

Dr. Richard Webby and other virologists are monitoring any mutations of the H5N1 virus to see if it becomes more of a threat to humans. (Photo: Courtesy of St. Jude Children’s Research Hospital)
WEBBY: Well, thank you so much. I've enjoyed it.
DOERING: Gosh, so, Aynsley, sounds like we'll have to keep an eagle eye on this bird flu story going forward.
O'NEILL: Definitely, but one thing that could complicate efforts to stay vigilant is the communication freeze that the Trump Administration put in place for health agencies including the Centers for Disease Control and Prevention.
DOERING: Hmm. And on day one, I believe, President Trump issued an executive order to pull the US out of the World Health Organization. So how might those moves imperil our ability to respond to a potential avian flu threat?
O’NEILL: Right, as you might imagine disease reporting that’s collected and disseminated by the CDC is essential to this country's disease response and control strategies. City and state health departments need to know if a new public health threat is starting to emerge, whether that’s avian flu or something else. Meanwhile, pulling out of the World Health Organization cuts off communication between the WHO and the CDC. You know, it takes a full year to officially withdraw – so we’re still on the hook for paying our membership dues of $130 million dollars – but even so, U.S. officials like those at the CDC have not been attending meetings at the WHO. As such, our researchers will be unable to examine the recent data from those gatherings on diseases like the avian flu.
DOERING: And the WHO has what, nearly 200 member states still? Sounds like we’ll be left out of the loop.
O’NEILL: We really could. And although many city, state, and nongovernmental organizations will be doing what they can to track disease numbers and spread, the exit from WHO and the pause on federal public health communication could lead to an epidemic spiraling out of control.
Links
Learn more about St. Jude Children’s Research Hospital.
Living on Earth wants to hear from you!
Living on Earth
62 Calef Highway, Suite 212
Lee, NH 03861
Telephone: 617-287-4121
E-mail: comments@loe.org
Newsletter [Click here]
Donate to Living on Earth!
Living on Earth is an independent media program and relies entirely on contributions from listeners and institutions supporting public service. Please donate now to preserve an independent environmental voice.
NewsletterLiving on Earth offers a weekly delivery of the show's rundown to your mailbox. Sign up for our newsletter today!
 Sailors For The Sea: Be the change you want to sea.
Sailors For The Sea: Be the change you want to sea.
 The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
 Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
 Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth
Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth