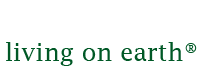May 19, 2017
Air Date: May 19, 2017
FULL SHOW
SEGMENTS

Air Pollution Chokes Out Happiness
View the page for this story
Nitrogen dioxide is known to harm health, especially lungs and heart, and now a new study finds that it can take a heavy toll on life satisfaction, too. Even if NO2 pollution is below the EU legal limit, sharp increases may lower life satisfaction as much as the death of a spouse. UK’s York University researcher Sarah Knight tells host Steve Curwood that parts of the UK far exceed the EU legal pollution limit, and what city-dwellers can do to protect themselves from this danger. (07:15)
Air Pollution Transformed into Renewable Energy
View the page for this story
The WHO reported in 2014 that over 90% of the world’s population live with polluted air. But now Belgian researchers using solar-powered nanomaterials have decontaminated small amounts of polluted air with a process that generates hydrogen at the same time. University of Antwerp researcher, Sammy Verbruggen, joins Steve Curwood to explain how their tiny photo-electrochemical prototype device shows promise, but to make an impact it needs to be scaled up. (06:35)

Science Note: The Power of Dust
/ Noble IngramView the page for this story
In the Sierra Nevada mountains, heavy runoff and erosion can steal precious soil nutrients from the ecosystem. New research shows replacements come from an unlikely source: dust flying in from far away, as Noble Ingram explains on this week’s Note on Emerging Science. (01:50)

Solar Eclipsing Coal in Jobs
/ Reid FrazierView the page for this story
Coal still produces much more energy in the U.S. than solar, which powers less than one and a half percent of the grid. Yet there are now twice as many solar jobs as those in coal. As the Allegheny Front’s Reid Frazier reports, some former coal miners are becoming solar technicians, though it may involve a pay cut. (05:30)

BirdNote: American Robins are Exceptional Singers
/ Michael SteinView the page for this story
The American Robin is often the first bird to sing in the morning, and the last bird to trill into the evening air. BirdNote’s Michael Stein describes how robins’s wide repertoire of caroling phrases and notes creates a unique serenade every time. (02:00)

Astrophysics for People in a Hurry
View the page for this story
Neil Degrasse Tyson directs New York’s Hayden Planetarium, but he’s also a Pop-Science rock star. Now he’s penned a slim volume titled Astrophysics For People In A Hurry, aiming to make his discipline accessible to non-scientists. He joined Living on Earth Host Steve Curwood for a flash-course on all things celestial, with topics ranging from spagettification by black hole, dark matter and energy, to how the periodic table encompasses, so far as we know, everything in the universe. (23:00)
Sounds of Space: The Songs Of Uranus And Neptune
View the page for this story
As they passed through the outer reaches of our solar system on the way to interstellar space, NASA’s twin Voyager spacecraft sent back data that include electromagnetic vibrations from Uranus and Neptune and other outer planets. NASA transformed that data into sounds we humans can hear, on its Symphonies of the Planets CD. (01:00)
Show Credits and Funders
Show Transcript
HOST: Steve Curwood
GUESTS: Sarah Knight, Sammy Verbruggen, Neil deGrasse Tyson
REPORTERS: Robert Frazier, Noble Ingram, Michael Stein
[THEME]
CURWOOD: From Public Radio International, this is Living on Earth.
[THEME]
CURWOOD: I’m Steve Curwood. Dirty air can not only damage hearts and lungs, it can also affect our happiness.
KNIGHT: We found that the loss in life satisfaction is something comparable to about 83% of marital separation; over 100% of widowhood, and just over 50% of unemployment. So, kind of three big things that really do affect your life satisfaction and your happiness.
CURWOOD: Also, space exploration isn’t just fun, it might save us from planetary disaster…
TYSON: Mars once had liquid running water, and it's bone-dry today. Something bad happened on Mars. We don't know what yet. Venus is 900 degrees Fahrenheit. It has a runaway greenhouse effect. So something bad happened on those planets. I wanna know what because I don't want that to happen to Earth.
CURWOOD: Neil deGrasse Tyson and more, this week on Living on Earth. Stick around!
[NEWSBREAK MUSIC: Boards Of Canada “Zoetrope” from “In A Beautiful Place Out In The Country” (Warp Records 2000)]
[THEME]
Air Pollution Chokes Out Happiness

Exposure to the air pollutant nitrogen dioxide at levels commonly seen in the U.K. may impact life satisfaction as much as being widowed. (Photo: Friends of the Earth Scotland/Maverick Photo Agency, Flickr CC BY 2.0)
CURWOOD: From PRI, and the Jennifer and Ted Stanley Studios at the University of Massachusetts Boston, this is Living on Earth. I’m Steve Curwood. Bad air is not only bad for your physical health. It’s also bad for your mental health and sense of well-being. That according to Researchers at York University in the UK, who recently reported that as nitrogen dioxide pollution increases, satisfaction and happiness decline. Their findings add to a body of literature that shows air pollution promotes health problems, including cardiovascular and respiratory diseases. Nitrogen dioxide is a byproduct of burning gasoline, diesel, and other fossil fuels and gets into the air through smokestacks and tailpipes. The lead author of the study is PhD candidate Sarah Knight, and she joins me now from York.
Welcome to Living on Earth.
KNIGHT: Hi there. Thanks for having me.
CURWOOD: So, your research looked at whether or not there is a link between nitrogen dioxide and life satisfaction. What did you find?
KNIGHT: Yeah, so we took a big dataset of over 50,000 adults in England, and this dataset is really nice because it tracks the same people through time and space. So, it's the same people being asked the same questions every year. So, we're able to bring that together with how air pollution changes through time and space as well and look at whether there were changes in life satisfaction and subjective well-being, and what proportion of that change is down to the changes in nitrogen dioxide.
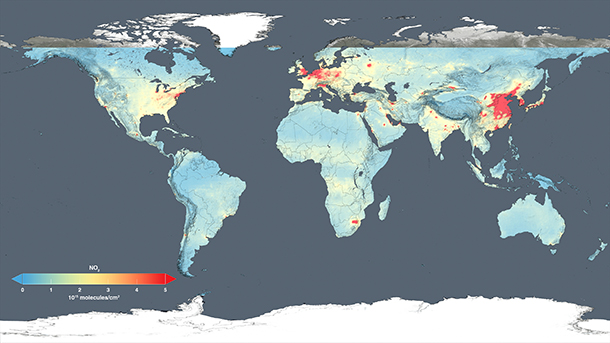
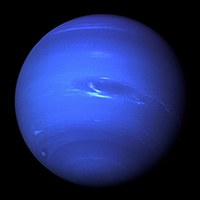
2014 NASA map of atmospheric nitrogen dioxide concentrations around the world. Note that the map shows concentrations from 0 to 5 quadrillion molecules -- that's an upper limit of 5 with 15 zeros after it! -- per centimeters squared. (Photo: NASA)
CURWOOD: OK, I can't wait to find out. What did you find in terms of a connection here?
KNIGHT: [LAUGHS] I'm glad you're so excited. The main results were an increase in 10 micrograms per meters cubed of nitrogen dioxide reduces your life satisfaction on a scale of one to seven by 0.03. [LAUGHS] So I don't know if any those numbers mean anything when you say it like that so we try to find other ways to communicate how meaningful that is for people. 0.03 on a scale of one to seven sounds quite small I guess. So, we wanted to compare it to the things that we know that reduce your happiness and well-being, things like your employment status and your relationship status. We found that if people are raised to a pollution level of the legal limit of 40 micrograms per meters cubed, the loss in life satisfaction is comparable to about 83% of marital separation, over 100% of widowhood and just over 50% of unemployment. So, kind of three big things that really do affect your life satisfaction, your happiness. Much more substantive than we were expecting.

Air pollution takes a toll on happiness, Sarah Knight conjectures, partly because of the stress of its physical effects. Nitrogen dioxide pollution irritates the lungs and is especially dangerous for people who have asthma or heart disease. (Photo: NIAID, Flickr CC BY 2.0)
CURWOOD: Now, there are a lot of other factors that can make people dissatisfied, of course. What other explanations did you consider?
KNIGHT: Absolutely. Yeah, so we try and account for as many of these kinds of things as we can. So we brought in all kinds of data into this. We brought in things around income. There's lots and lots of explanation about how income is related to our well-being. We also brought in other geographical factors as well, so other things about the neighborhood that you live in as well. So, things like population density, crime, education, income deprivation, isolation as well - so how close you are to services and your GP and health services and things like that. We also looked to account for other environmental factors as well, like green space and blue space. So, we tried to hold all those things constant and then tried and isolate that effect that pollution will have on your life satisfaction.
CURWOOD: So, what are the pollution levels of nitrogen dioxide there in the UK?

London, shown above on a smoggy day, regularly exceeds its annual nitrogen dioxide limit in the first week of the year. This year, it breached the legal limit in just five days. (Photo: Chris LL, Wikimedia Commons CC BY-SA 2.0)
KNIGHT: So, air pollution is in the news a lot at the moment, definitely here in the UK, and nitrogen dioxide specifically. We looked at where the highest and lowest values were within England and we saw that the average value somewhere down in Cornwall and Devon was something like three micrograms per meters cubed, and in London the average was something like 58, 60. So, you get this huge geographic difference in average values. So, 10 micrograms per meters cubed is quite a big jump. The legal limit set by the EU based on World Health Organization guidelines is 40 micrograms per meters cubed. So, that gives you some kind of measure of what's high and what's low. Actually, although most parts of the UK are below that, but there are kind of hotspots where you get this fluctuation above that point. So, there’s two limits. There's the annual exceedance limit, and there's also these daily limits as well that are set, and within London it was exceeded within the first five days of the year this year. It's pretty much the same every year. It's exceeded within the first week for nitrogen dioxide within London, and the UK government has been taken to court over it.
CURWOOD: Now, what you think is the mechanism that operates here when nitrogen dioxide impacts life satisfaction? What's going on?
KNIGHT: Yeah, that's a really good question, and it's way out of the scope of our study that we could do, but through speaking to lots of people about this analysis and reading through the literature, we've kind of come up with four different mechanisms that will influence this relationship.
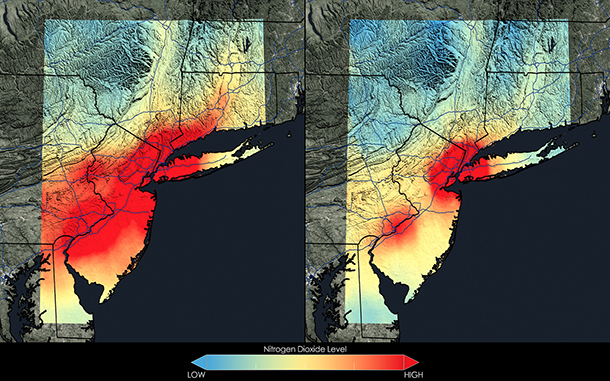
Here in the United States, local nitrogen dioxide hotspots have improved dramatically in recent years. Above, satellite data from NASA shows that the New York City metropolitan area saw a 32 percent decrease in NO2 between the 2005-2007 and 2009-2011 periods. (Photo: NASA)
So, we think firstly and probably by far the most important is through health. So, the way that nitrogen dioxide influences people with existing health conditions and exacerbates and brings on new conditions. So it's been related to cardiovascular and respiratory issues. The second one would be how pollution influences your mental and cognitive health and that kind of development. So, there's been links to things like dementia. So, two very strongly kind of health points. The third we would say definitely through aesthetics - so the look, the smell, the taste of your city and your neighborhood - what it looks like if you see it. Just that kind of walking down the street and you smell that really will impact you and the day that you're having. And then lastly we've been speaking to lots of people just out and about at different public events about this and many people talk about the concern that they have for their family, their children and their own health and more broadly for the environment as well. So, you know, they see this pollution. They smell this pollution. They think, gosh, what's this doing to myself and my children?

City-dwellers can dramatically lower their exposure to nitrogen dioxide by choosing bicycling and walking routes away from congested areas. The air is typically much cleaner just one street over from a high-traffic thoroughfare. (Photo: Leo Hidalgo, Flickr CC BY-NC 2.0)
CURWOOD: When you speak to people about this, sometimes informally, what is the one or two things that people really want to know?
KNIGHT: There's two main things that people are concerned about and where we've started having conversations with people out and about. The first one is, people are concerned about either what fuel should they be using, as consumers, what decisions should they be making, and they feel they've been getting mixed information over the past few years. The other one is about where the hotspots are and how they can avoid it.

Sarah Knight is a PhD candidate at the University of York.
CURWOOD: So, what do you tell people about the hotspots? Where should people avoid if they're concerned about this?
KNIGHT: Certainly central London, as you would expect. some other large cities around the UK and Heathrow airport as well. So it would be these major centers of traffic and transport. But certainly, you know you're definitely less exposed even if you're just one road off the main road, as it were, in these areas, as it were, so even if you can find kind of quieter routes to where you want to go and cycle and walk those, you're really reducing your exposure to traffic air pollution significantly.
CURWOOD: Sarah Knight is a PhD candidate at the University of York where she studies the impact of the environment on us humans. Thank you so much, Sarah, for taking the time with us today.
KNIGHT: Thank you for having me.
Related links:
- Read the study: “Can clean air make you happy? Examining the effect of nitrogen dioxide (NO2) on life satisfaction”
- The Guardian: “Air pollution as bad for wellbeing as partner’s death, say researchers”
- EPA info on Nitrogen Dioxide (NO2) Pollution
- Editorial from The Guardian: “The Guardian view on May’s air pollution plan: all mouth, no trousers”
- London Air Quality Network’s Annual Pollution Maps
Air Pollution Transformed into Renewable Energy
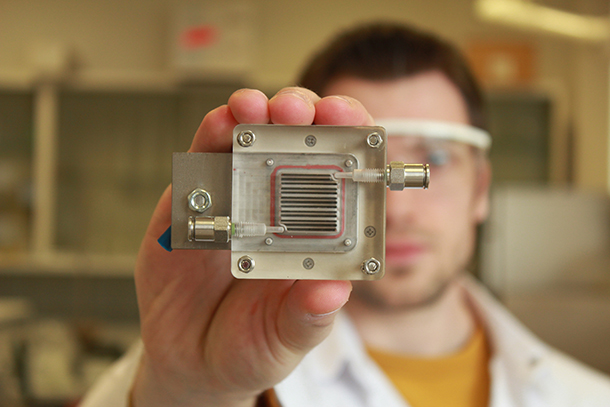
The Photoelectrochemical (PEC) Cell currently has an active area of only about one square centimeter. Sammy Verbruggen and his colleagues are working on scaling up the device. (Photo: KU Leuven News)
CURWOOD: Well, the world is awash in polluted air these days. Over 90 percent of us breathe it in every day, and as we have just heard at least one of those pollutants robs us of some happiness.
But with this bad news comes good news from the world of technology, as a team of Belgian researchers has developed a way to clean the air of some pollutants and convert them into simple hydrogen, using sunlight, special membranes and nanoparticles. Hydrogen is a clean industrial chemical and, when burned, simply makes water.
Sammy Verbruggen is a researcher from the University of Antwerp and part of the team that achieved this breakthrough, so we called him up to ask how it works. Sammy Verbruggen, welcome to Living on Earth.
VERBRUGGEN: Thanks, Steve.
CURWOOD: So, tell me. How was this project conceived and who were the primary players in developing this study?
VERBRUGGEN: Well, actually it's in two parts. So, for the one part, our group in Antwerp has been working a lot on trying to do air purification using light. So, we're developing a special type of nanomaterials called photocatalysts. So, nanomaterials that are activated by light and that way you can convert air pollution in less toxic compounds. Now, on the other hand there's the group at Leuven University where Professor Johan Martus is working a lot on these photo electro chemical cells. So, he's actually working on these membranes and devices to try to convert water into hydrogen gas. And now in this step, we actually try to combine both processes together and try to use not water, but actually polluted air to convert it to hydrogen.

The city of London began a trial of hydrogen-powered buses like the one above in 2011. According to Verbruggen, hydrogen power’s most common applications today are in vehicles. (Photo: mangopulp2008, Flickr CC BY-NC-ND 2.0)
CURWOOD: Explain for us just how this air purifying process works now.
VERBRUGGEN: Well, at first instance you have the nanomaterial. It's activated by light, so you get charges inside of this semiconductor. Well, the next step is that these charges, they will attack some of the molecules that are adsorbed on the surface of the material, and that way you get very active oxygen species. We actually do an oxidation. So, you oxidize organic molecules until, when they're completely mineralized, you end up with CO2. The hydrogen is something very specific. During these degradation reactions, you also get some protons coming out of the molecules, and these protons, they go through a membrane to the other side of the device where another catalyst is present and it will reduce the protons to hydrogen gas.
CURWOOD: So, what kind of pollutants can you filter with this device, and how clean is the air once it's done purifying it?
VERBRUGGEN: Well, at first instance, we have been looking at quite simple, small, organic molecules, so our study primarily focuses on methanol, also in part, to acetic acid and some ethanol. So, fairly small organic molecules. But still, some of these molecules can be quite hazardous to your health as well, and for instance, formaldehyde, is a very known indoor air pollutant which can cause you headaches. Acetaldehyde, it's the stuff in your brain that gives you a hangover, so obviously they're not very healthy to you. So, attacking these small molecules is quite useful as well.
Ideally, everything that passes through the light side of the device gets oxidized to CO2, so to give you a very straightforward example, let's say we start from methane. So it's one carbon atom and four hydrogen atoms . In the most ideal case it will end up as one CO2 molecule at one side and two hydrogen gas molecules on the other side. Obviously, efficiencies aren't 100 percent as the device is proposed right now, but that's something we are working on.

Solar panels capture energy in Cariñena, Spain. Since the photochemical cell requires ultraviolet light to function and Verbruggen’s home country is far cloudier than the Mediterranean, he and his colleagues are working on improving the technology’s efficiency in regions with less visible light. (Photo: Diego Delso, Flickr CC BY-SA 2.0)
CURWOOD: So, Sammy, we all hear about, “Oh, this could be a great technology.” How optimistic are you about the possible implementation of what you have put together? I mean, how difficult is this going to be to increase this to a scale that will be useful?
VERBRUGGEN: It will certainly be challenging. There's no denying that. I think we're facing two major issues that have to be resolved right now to get it to a breakthrough level. One thing is to increase the efficiency. These materials that we are using, they're actually activated by UV light right now. So, if you have a look at the solar spectrum, the contribution of UV light is at most five percent of the total spectrum.
Now, what we are trying on one hand is to modify these materials so they can use visible light. So, in that way, we could extend our activity range, let's say, to 40, even 50 percent of the solar spectrum. Another thing would be to try to upscale this design, because in the first test we have been doing now, we are working on a membrane with an active area of about one square centimeter, which is obviously not much. If you compare this to other solar-driven technologies like solar panels you can put up on your roof of your house, we're looking at square meters of active area. So the upscaling towards larger membrane sizes will be the other challenge.
CURWOOD: So, talk to me about the amount of sunlight required to facilitate the process. You say it has to be ultraviolet, it has to be in the ultraviolet spectrum right now. How useful would this be, do you think, if it were to be cloudier?
VERBRUGGEN: Absolutely, and there's also the geographic position of the country you would like to use it in. I live in Belgium, and it's very optimistic to think you can drive everything on solar energy because it's indeed quite cloudy here most of the time. Whereas, if you look at real pilot scales of photocatalytic processes, they're mostly centered around the Mediterranean here in Europe or even in Japan there's also some applications of that. So, that's indeed a very good point. That's why we are also working on modifying these materials towards visible light because that's always around.

Sammy Verbruggen is a guest professor and postdoctoral fellow studying photocatalysis for environmental and energy applications at the University of Antwerp. (Photo: Antwerp, Smart Region Link)
CURWOOD: So, how big a deal does this have to become, Sammy, to make a significant dent on urban air quality? I mean, how possible would it be for your devices to clean up the smoggy air of, say, Paris or Beijing or Mumbai? There are a lot of places with a lot of dirty air.
VERBRUGGEN: Yeah, that's true. But I think it's a little utopia to think we can clean up an entire city. That's maybe a little far-fetched. But I think it might come in handy in conjunction with a very specific industrial process, for instance. I can imagine if you're in the paint industry or something, in textiles, that there are some processes involving a lot of organic solvents. So, I can imagine they have very loaded waste streams loaded with organic molecules, and they will have to meet some environmental quota. They can't just put in the air, or I hope they don't just put it in the air. So, if they could use a technology like this one, they can hit two flies at once. They could purify the air on one hand, but on the other hand, they can recover part of the energy that is stored in this polluted air as hydrogen gas, for instance.
CURWOOD: Sammy Verbruggen is a guest professor and post-doctoral fellow studying photocatalysis for environmental and energy applications at the University of Antwerp in Belgium. Thanks so much, Sammy, for taking the time with us today.
VERBRUGGEN: You’re welcome.
Related links:
- KU Leuven Press Release
- University of Antwerp Photocatalysis Research
- KU Leuven Energy Research
[MUSIC: Tommy Dorsey and His Orchestra, “Smoke Gets In Your Eyes” on The Best of Jerome Kern, Kern/Harburg, Chestnut Records]
CURWOOD: Coming up, why tiny dust particles can be a big help for mountains. Stay tuned to Living on Earth.
ANNOUNCER: Support for Living on Earth comes from the Gordon and Betty Moore Foundation, and from a friend of Sailors for the Sea, working with boaters to restore ocean health.
[CUTAWAY MUSIC: Bobby Timmons, “Dat Dere” on This Here Is Bobby Timmons, Riverside Records.]
Science Note: The Power of Dust

Ecosystems with intense erosion, like the Sierra Nevada mountains, can benefit greatly from the minerals in airborne dust. (Photo: Don Graham, Flickr CC BY-SA 2.0)
CURWOOD: It’s Living on Earth. I’m Steve Curwood. In a minute, a handy guide to the mysteries of the universe, but first this note on emerging science from Noble Ingram.
[SCIENCE NOTE THEME]
INGRAM: If you’re looking to do some spring cleaning, it can be a big nuisance. But ask any gardener, and you may be surprised to hear that dust is good for more than just collecting in odd corners. A research team from several Western and Midwestern universities has shown that rock dust plays a critical role in feeding the soil of the Sierra Nevada mountains.
Older mountain ranges, like the Sierra Nevadas, experience heavy rainfall and intense erosion, costing them significant soil nutrients in runoff. In California, dust rich in plant-boosting elements like phosphorous, offers the perfect refreshment to these weathered environments.
Air currents collect the nutrient-dense cargo from deserts and dry valleys and carry it vast distances. Researchers found that between 18% and 45% of deposits in the Sierra Nevadas came from Asia, crossing the Pacific Ocean. Some dust was even detected from the Gobi Desert on the border of China and Mongolia, more than 6,000 miles away.
Despite its beneficial effects, this mineral mobility leaves some scientists worried. Bounties of imported nutrients may help a forest thrive, but they can wreak havoc on surrounding rivers and lakes. Dust-fed algae blooms suck up oxygen and threaten to suffocate fish and crustaceans.
With drought spreading as climate change advances, geologists suspect dustier forecasts are ahead. And cleaning up the potential ecological upsets in their wake will require more than a feather duster.
That’s this week’s note on emerging science. I’m Noble Ingram.
Related links:
- EurekAlert! press release: “Dust contributes valuable nutrients to Sierra Nevada forest ecosystems”
- The Study in Nature Communications: “Dust outpaces bedrock in nutrient supply to montane forest ecosystems”
[SCIENCE NOTE THEME]
Solar Eclipsing Coal in Jobs

Craig Williams, a miner at Consol Energy’s Harvey Mine in Sycamore, PA. (Photo: Reid Frazier)
CURWOOD: Across America, the numbers of coal miners are declining while the ranks of renewable energy workers continue to swell, but aggregate numbers don’t tell the local stories. So the Allegheny Front’s Reid Frazier set out to check the impact of solar jobs in the heart of Pennsylvania’s coal country. Here’s his report.
FRAZIER: At Consol Energy’s Harvey mine--about an hour south of Pittsburgh--Craig Williams takes a mid-day break above ground, in a big room at the mine’s entrance. He’s in a hard hat, and he’s wearing a dusty, yellow-striped mining jacket. Though times have been tough in the coal industry, Williams and other miners here have managed to keep their jobs. He hopes to hang onto his job as a foreman.
WILLIAMS: Pittsburgh used to be big on steel. That’s mostly gone now. We’re one of the last, you know, industries around and hope to keep it that way.
FRAZIER: A father of two, he says, like generations before him, coal is the best way he’s able to support his family. Williams wouldn’t say how much he’s making, but nationwide coal miners make around $80 thousand a year.
WILLIAMS: If you had to take another job, in this area especially, you’re going to take anywhere from a 50 to 70 percent pay cut to what the next best thing that’s out there.
FRAZIER: But 40 percent of coal mining jobs have disappeared since 2011, and now only 50,000 of these jobs remain. Experts say automation, lower demand for electricity, and above all competition from cheaper fuels are what’s killing the industry. Those fuels include natural gas from fracking, and--increasingly--renewable energy.
Solar power now accounts for just under one and a half percent of electricity in the U.S. But according to the Department of Energy, Solar jobs now outnumber those in coal by more than 2 to 1. So, can laid off coal miners find jobs in solar?
GODBY: Well certainly it's a possibility, but there are a couple of major challenges.

Solar jobs now outnumber coal jobs 2 to 1. But are laid off miners getting them? (Photo: Reid Frazier)
FRAZIER: Rob Godby is an energy economist at the University of Wyoming. He says one of those challenges is simple. Location.
GODBY: When you are thinking about coal mining in Appalachia, I mean, oftentimes there are generations of families in those regions, and it's just very difficult to pick up and move.
FRAZIER: And then, there’s pay. Coal miners make on average around $35 an hour, Godby says, in part because the job can be so dangerous. In renewables, the pay is more like $20 and $25 an hour.
GODBY: That doesn't mean you couldn't raise a family on that, but you're a lot closer to the average income in a lot of states in the solar industry than you are in mining industries.
[SOUND OF CONSTRUCTION]
FRAZIER: On a rooftop at a community center in Millvale, just outside of Pittsburgh, a gang of seven workers in neon green t-shirts bolt down solar panels. The crew includes 23-year-old Wylie Koontz, who worked at a coal mine, though as a lower paid contractor.
When he got laid off last year, he saw a job opening with Energy Independent Solutions, a local solar company.
KOONTZ: I didn’t like, just mentally be like, I’m going to completely switch. It was a coincidence I just found this job it was about the same pay rate that I was making before, so I just went for it. Ended up really likin' it.
FRAZIER: One thing that did not weigh on his decision: Climate change.
KOONTZ: “I don’t believe in the whole climate change thing or not. I mean, it good. It does takes the carbon for sure. It's a lot less, and it's a lot cleaner energy...
FRAZIER: But a steady paycheck, not necessarily the environment, is what attracted him to the job. Koontz says he likes the problem-solving he gets to do in solar, and he could see himself staying in the industry.
Brian Krenzelak was a roofer before joining the company seven and a half years ago.

Workers for Energy Independent Solutions install solar panels at a Millvale, PA community center. (Photo: Reid Frazier)
[SOUND OF INSTALLING PANELS]
FRAZIER: Krenzelak installs solar and trains others, and he believes in the mission of green energy. His wife also works, and the couple have three kids, one in college, with another about to enter. They even have a second home in Florida.
KRENZELAK: You know, I do well with this company, very well. It covers what we need to cover. I mean, I'm not becoming a millionaire overnight but steadily have been building the nest egg for sure.
FRAZIER: Experts say solar’s boom is being helped by incentives that are schedule to run out in a few years. And they don’t know how long the surge in business will last. Some companies are reporting lower installations nationwide. But solar jobs in Pennsylvania grew last year by almost a quarter. And Krenzelak’s company, Energy Independent Solutions, is busy. Its looking to basically double its workforce of 22 over the next year and a half.
KRENZELAK: We had to slow the salesmen down at a certain point. We were selling so much solar. We had to actually slow them down a little bit until we caught up.
FRAZIER: Krenzelak likes the idea that his industry is up and coming. He says, when his crew went on a big installation recently, other contractors were curious about what they do.
KRENZELAK: So, there was iron workers there, carpenters, union laborers. Everyone’s coming up to us.
FRAZIER: He says it feels like solar is an industry of the future. And when he looks into the future, he likes what he sees. I’m Reid Frazier.
CURWOOD: Reid Frazier reports for the Pennsylvania public radio program, the Allegheny Front.
Related links:
- Listen to the story on the Allegheny Front website
- Annual coal mining wages stats from the National Mining Association
- “Can Coal Make a Comeback?” – Report from the Center on Global Energy Policy
- NYTimes: “Today’s Energy Jobs Are in Solar, Not Coal”
BirdNote: American Robins are Exceptional Singers

An American Robin singing (Photo: Gary Witt)
[MUSIC - BIRDNOTE® THEME]
CURWOOD: Of all the sounds of early summer that make us think of long sun-filled days ahead, the chorus of birds singing even before dawn are among the most joyful. And as Michael Stein points out in today’s BirdNote, there’s one showy stand-out.
[AMERICAN ROBIN SONG]
STEIN: These rich, caroled phrases are among the best loved and most widely heard in North America. This superb song belongs to perhaps our most familiar bird, the American Robin.
As singers go, the robin is exceptional. They’re often the first birds to sing in the morning, starting well before dawn,
[WHINNY]
and the last you’ll hear in the evening, holding forth into deep twilight.
[CONTINUOUS ROBIN SONG IN BACKGROUND]
Robins also begin singing earlier in the year than most birds, often in mid-winter.
[CONTINUOUS ROBIN SONG IN BACKGROUND]

A robin sings from a nest perched in a conifer (Photo: Tom Grey)
And robins can be remarkably long-winded. While their average song strings fewer than a dozen short phrases together and lasts a few seconds, robins sometimes sing for minutes without a pause.
[ROBIN SONG CONTINUES.]
But the most extraordinary measure of robin song is its variety. In each song, a robin sings a varied selection from a repertoire of 10 to 20 different caroling phrases. Then, as evening comes on, the same robin interweaves these phrases with exquisite whisper-like notes from its personal treasury of 75 to 100 different whispered notes.
[SONG INCORPORATING HISSELLY PHRASES]
The potential song variety is amazing. The musical results, enchanting. I’m Michael Stein.
Written by Bob Sundstrom
Bird sounds provided by The Macaulay Library of Natural Sounds at the Cornell Lab of Ornithology, Ithaca, New York. Song of American Robin [94255] recorded by W.L. Hershberger; [44903] recorded by G.A.Keller; song with hisselly phrases [94383] recorded by W.L. Hershberger.
Producer: John Kessler
Executive Producer: Chris Peterson
© 2005-2017 Tune In to Nature.org May 2017 Narrator: Michael Stein
Background on repertoire and variation from Donald Kroodsma’s “The Singing Life of Birds,” 2005 and from Nathan Pieplow’s “A Robin’s Many Songs” at http://earbirding.com/blog/archives/2836
CURWOOD: And for photos, soar on over to our website, LOE.org.
Related links:
- Listen on the BirdNote website
- About the American Robin, from the Cornell Lab of Ornithology
[MUSIC The American Café Orchestra, “Hell Broke Loose In Georgia” on Egyptian Dominoes, Traditional American/arr. M. Alfred, Northeastern Records]
Astrophysics for People in a Hurry

The Andromeda galaxy is a spiral like our Milky Way, and it’s also the closest major galaxy to our own. (Photo: NASA/JPL-Caltech)
CURWOOD: If you ask Neil deGrasse Tyson, astrophysics is a lot of fun, even funny at times, but not all of us love physics. Maybe because it uses daunting mathematical formulae, or things we know are dangerous but can’t see, such as x-rays and nuclear radiation. Still, most us do enjoy hearing about the latest NASA probe to fly by a distant planet or seeing the latest pictures from the Hubble telescope or the Mars rover.
So Neil, who is the most popular populist of all things celestial as well as director of New York’s Hayden Planetarium, has written a book that’s perfect for those of us with a shaky understanding of the mysteries and marvels of the universe. It’s called "Astrophysics for People in a Hurry". We haven’t talked recently since he’s been busy with everything from the Cosmos TV series to podcasting, so with his new book we thought we could call him up to get the low-down on things way high up.
Neil deGrasse Tyson, welcome back to Living on Earth.
TYSON: Hey, it’s been a while! You don't call, you don't write you don't...[LAUGHS]
CURWOOD: I know. We'll have to have you back on a more regular fashion here. So, I've got to start by asking you what compelled you to write a book that explains astrophysics in this way. Astrophysics for people in a hurry.
TYSON: Well, I noticed that there were people who were assembling fragmented bits of the universe that they get from the evening news or from a newspaper headline or from some chatter of the water cooler, and there was no mechanism to render these fragments of information coherent. What I did was to create this book, "Astrophysics for People in a Hurry,” on the premise that not everyone has the time to go read a text book or to look at a long video series on television. But you still want to be fluent. You still want to be able to have that conversation about the multi-verse or about the big bang or about Pluto as no longer a planet or colliding black holes or wormholes. You want to be able to still have that conversation. So, this book was written with you in mind.

Astrophysics for People in a Hurry is Neil deGrasse Tyson’s latest book. (Photo: W.W. Norton & Company)
CURWOOD: And I have to say that, if you are in a hurry, it doesn't take that long to get through. It took me maybe a little less than two hours, actually.
TYSON: Oh cool. It took me about three hours. I think I'm a slow reader than you.
CURWOOD: Well, you have to look to see if there are anything that need editing.
TYSON: [LAUGHS]
CURWOOD: I just have to look and say, "Oh yeah," and laugh sometimes. A lot of laughter in this book, here.
TYSON: Well, I think the universe is fundamentally a funny place I would be disingenuous if I described the universe without that hilarity.
CURWOOD: Oh, really? What's the funniest part of about the universe?
TYSON: Just…[LAUGHS]…Well, I think the funniest part is…Well, it's morbidly funny, and I described it in a separate book, a whole other book called "Death by Black Hole". I just think it's kind of hilarious how you would die if you fell into a black hole because you wouldn't just get sort of squashed by the gravity upon landing. En route you get stretched as the tidal forces of gravity pull your feet faster than your head. This is in a feet first dive. So you end up stretching. But not only that, you're getting funneled through the fabric of space and time, so that you end up getting extruded, literally extruded through space like toothpaste through a tube, and you end up diving into the center of the black hole in a long stream of atoms.
CURWOOD: Like a piece of spaghetti, huh?
TYSON: Yeah that’s where you get the word. This is the process of spaghettification.
CURWOOD: [LAUGHS] So, your book begins with the very basics of astrophysics. You march us through from the big bang that got our universe going, and then you get to the point that is not explainable; that is, these forces of gravity that we really have no explanation for except that they're obviously there. You use the title “Dark Matter,” and then you talk about “Dark Energy.” There’s more of that than there is of what we know. So, tell me what we know about what we don't know.
TYSON: No, it's one thing to not know what you're talking about. It's another thing to know that you don't know what you're talking about. These are two separate things. So, in that, it's kind of fun to learn how we came to realize and quantify what it is we don't know. In "Astrophysics for People in a Hurry", two of the dozen chapters are just what you described, one on dark matter and one on dark energy. We have no idea what either of those things are, but we can measure their existence. So we know there's something out there that yields to our measurements but has yet to yield to our creative explanations of what the hell the stuff is. And when you add them together, they comprise 95 percent of all that drives the universe. So, in other words, everything we know and love and all the forces we've measured and understand, and the physics, the chemistry, the biology, the geology, everything is contained within the five percent of the universe that we understand, we can predict, we build courses around it, and the rest is unknown.
CURWOOD: Now, your book discusses the periodic table.
A NASA black hole simulation video.
TYSON: Oh, yeah, I had to put it in there because I love me some periodic table of the elements, and I know it gave some people the shakes because they remember it from high school chemistry, but I just thought it was time to have fun with it, and there's a lot of fun you can have on that table.
CURWOOD: What does it have to do with the evolution of the universe?
TYSON: Well, yeah. So, in this table of 92 elements, plus some extra that we invented, everything in the universe that has material substance is found on this table. Every atom in the universe is there. So, what is Earth made of? It's made of three of these and four of those and five of those and then stir, shake, not stir, whatever. You can reconstruct the universe and then you can ask where these elements come from.
I once asked that of my high school chemistry teacher, and he said, "They come from the Earth," and I said, "OK, but where did they come from before they were in the Earth?" He couldn't answer that question. Well he could, but the answer doesn't come from chemistry. It comes from astrophysics because we learned at mid-century, mid-20th century, where these elements came from. There are forged in the crucibles of high mass, high temperature stars where light elements like hydrogen and helium and carbon combine to become heavier elements, and then that's not good enough because then they’d just be stuck inside the star. What the star then does is, it explodes, scattering its enriched guts across the galaxy, enabling interesting things to form like planets and petunias and people, right? So, the periodic table of elements is an excuse to talk about the formation of the elements in the context of astrophysics and have a little bit of fun along the way.
CURWOOD: That's Neil deGrasse Tyson on his new book, "Astrophysics for People in a Hurry". He'll be back after a break. This is Living on Earth. Stay tuned.
[MUSIC: “ Paul McCartney & Wings, “Venus And Mars” on Venus And Mars, Capitol Records 1975]
ANNOUNCER: Funding for Living on Earth comes from you our listeners, and United Technologies - combining passion for science with engineering to create solutions designed for sustainability in the aerospace, food refrigeration and building industries. UTC companies such as Otis, Carrier, Pratt & Whitney and UTC Aerospace Systems are helping to move the world forward.
This is PRI, Public Radio International.
[CUTAWAY MUSIC: London Symphony Orchestra, “Venus, Bringer of Peace” from “The Planets,” Geoffrey Simon conductor on HOLST: The Planets, composed by Gustav Holst, Philips Records]
CURWOOD: It’s Living on Earth, I’m Steve Curwood. And we’re back now with Neil deGrasse Tyson, talking about his new book, "Astrophysics for People in a Hurry".
So Neil, there's another interesting observation you make. Why are spheres so prevalent in the universe?
TYSON: Oh spheres, I love spheres! There's a chapter in there on being round, and which celebrates the laws of physics as they manifest in the universe, where they give preference to things being round. It's that simple. And, you know, soap bubbles are round, planets are round, stars are round, stars are practically perfect spheres.
Now, here's what happens. If you spin fast, you flatten a little bit. You get a little wider in your belly than pole to pole. So, by looking at how flattened something is, from a sphere, you can then calculate how fast the thing must be rotating.

A panoramic view of the Milky Way. (Photo: Lukas Schlagenhauf, Flickr CC BY-ND 2.0)
CURWOOD: So, here's my question. The Milky Way, circular and very flat. What happened?
TYSON: Yes, it's really, really flat. It's flat as a crepe. I would say it's as a flat as a flapjack, but it's even flatter than that. So, here's the Milky Way wanting to be a sphere, but what happens is the gas - I'm talking about the early history - the gas collapses. It sticks to itself like two hot marshmallows at the equatorial plane of its rotation, and so what you get is this gaseous disc. But we have stars scattered above and below the disc that formed before any of that happened. We call that the galactic bulge, and so, yes, you learn about what was left behind in the spherical shape.
So, you get flat galaxies and we have some puffy galaxies, too, because they have less gas where they formed the stars before the gas collapsed, and they stayed in the spherical shapes. So, if you look at the inventory of galaxies in the universe, you know, there's a whole population of them that are spherical, and so our galaxy is about 12 billion years old, something like that, formed shortly after the beginning of the universe. Our solar system formed much later. Our solar system formed late enough for the cloud out of which we collapsed to have contained the enrichment from these high-mass stars that made silicon and nitrogen and oxygen, and all the good things that comprise life. Had Earth formed very early in the solar system, it's likely we would not had enough ingredients to make life as we know it.
CURWOOD: So, speaking of the formation of Earth, we're always excited when there's any news of any other place that could be like Earth - an exoplanet, an exosolar planet, as these guys sometimes call them. Talk to me about the recent ones that we've seen and which ones are most exciting. Which ones, if you could explore, where would you go?
TYSON: Yeah, our catalogs report rising through 3,000 exoplanets, planets orbiting stars not our sun. And what's kind of cool about that is, this whole thing began in 1995. So, recently there's a set of seven planets discovered around a star system called Trappist II, I think it is, and there's seven stars, each of them Earthlike, three of them orbiting the famous Goldilocks Zone. You want to orbit too close to your host star because if you have liquid water there it would evaporate and too far away it would freeze. Life as we know it on Earth thrives in liquid water, so if you're looking for life as we know it, you're going to look for life in the Goldilocks Zone. So this has three planets out of those seven orbiting in the Goldilocks Zone. So this made headlines justifiably.

Neil deGrasse Tyson speaking on a visit with Goddard's Space Flight Center Director Chris Scolese and the James Webb Space Telescope team. (Photo: NASA Goddard Spaceflight Center, Flickr CC BY 2.0)
But I want to caution that while there are Earth-sized planets, I wouldn't call them Earthlike planets because how long does it take Earth to orbit our sun? Tell me.
CURWOOD: It takes roughly a year. That's what call “a year.”
TYSON: Thank you. 365 and a little bit of days, OK. You know how many days it takes these planets to orbit? Six days, eight days to go around their host star. It's a very different dynamical system.
CURWOOD: Huh. Where in the universe does look like Earth?
TYSON: We've got a few. So, of the 3,000 exoplanets, I forgot the number, a couple hundred maybe, are Earthlike planets around a Goldilocks Zone orbiting a sunlike star. That's the difference. The Trappist star system is not a sun-like star. It's a much cooler star. Much much cooler. That's why the planets have to orbit close in to get the Goldilocks Zone happening for them.
CURWOOD: Speaking of Earthlike places, where in the 'hood, where nearby in our solar system are there some moons that might be attractive for Earth-type activities?
TYSON: We're pretty sure there's no other sign of intelligent life in our solar system. Some would argue there's no intelligent life on Earth. But in our solar system there are half a dozen places that are tantalizing for the possible thriving of microbial life, possibly deep under the soils of Mars, where we know there is water down there. Is there a place that keeps it warm? Radioactive decay is a source of heat. We know some elements stay radioactive for long times. Could there be aquifers, liquid underground puddles of water where microbes are just thriving?
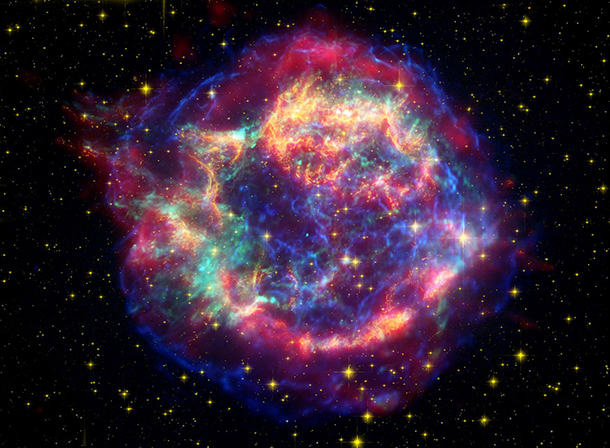
Supernova Cassiopeia A. Life as we know it is possible in part because some stars explode spectacularly at the end of their life, and these supernovae incubate as well as disperse throughout the universe the heavier elements necessary to life. (Photo: NASA/JPL-Caltech/STScI/CXC/SAO)
There’s Jupiter's moon Europa, well, out of the Goldilocks Zone, but it is kept warm through these stress forces induced by Jupiter and other surrounding moons. So, this place is, on its surface, solid ice, but the heat from Jupiter is getting pumped in deep within the center, and we are nearly certain that this ice is afloat atop of an ocean of liquid water that's been liquid for billions of years. So, I joke about this. I want to go ice fishing on Europa, go cut a hole through the ice, put down a submersible, see if anything swims up to the camera lens and licks it. Then you're good.
CURWOOD: So, in writing "Astrophysics for People in a Hurry", you demonstrate your ability to explain things in terms that average people can grasp and even enjoy and have fun. So, what's your process for doing that with a complex target?
TYSON: Yeah, thanks for that question. If I come to the table equipped with a utility belt of pop culture references, and you come to the table with your pop culture references, of course, you have the same references as I do. That's why it's called pop culture. OK? Now, you might be a different subset of pop culture, tbut hat's my duty to then learn what that is. OK? So, you come for the pop culture and you stay for the science, and this is not dumbing it down. I don't think I've ever been accused of dumbing down science. It's simply finding other interesting ways to think about science, and I'll give an example.
I'm watching a football game. I was channel surfing. I had 15 minutes until a movie I wanted to watch, and I came upon a football game that in that moment went into overtime.
A view of planet Earth. (Photo: NOAA/NASA GOES Project)
CURWOOD: Uh-oh.
TYSON: And, sure enough, one team got possession of the ball, and then they kicked a 50-some-odd yard field goal. And I'm looking at it, and the ball tumbles, and then it goes and it hits the left upright and then careens through for the win. And I said, "Wait a minute", and I checked the orientation of the stadium. I looked at how far the ball was kicked, and then I tweeted. I said, "The winning kick..." - I think it was the Cincinati Bengals - "was aided by a third of an inch shift to the right due to the rotation of the Earth".
CURWOOD: [LAUGHS]
TYSON: Oh my God. It blew people's minds!And it was, like, “Oh my gosh, the Earth helped!” And then, people got all, “God helped the Cincinati Bengals win the game”. And I didn't have to explain to you what a field goal was or what football is or what a left upright is. That's the common language of pop culture. And now I add something to it. You know, you're going to be interested in that. You know it because it's kind of weird and it's fun to know, even if you're on the losing end of that three point goal.
So that’s an example. And I found that when you do that people want more.
CURWOOD: So, by the way, in what ways if any has the current political climate impacted how you communicate science these days?
TYSON: Yeah, you know, stuff just got real, right, in the last several months? So, it's one thing to say whatever you want as a politician running for office, but if you then attain office and then enact it, then…Because politicians will say anything to get elected by and large, so you can pander but then reality has to set in when you want to pass an actual law, and it's important that laws, to the extent that science matters to them, that they're based on actual science.
I don't know when you'll air this, but we are in the time of the science march on Washington scheduled to coincide with Earth Day, and many scientists are coming out for this, just to show importance of the role and value of science in enlightened governance.
By the way, Abraham Lincoln, in 1863, when he clearly had more important things on his plate, in that year signed into law the National Academy of Sciences which then and now is charged with…It's an independent party of research scientists who are charged with advising the government on all ways science matters to policy. Abraham Lincoln was a Republican, by the way, in case people forgot. So, you go through the years of presidents and you find these major moments where agencies were established to exploit the role that science can and should play in our health, our wealth, and our security. If you turn around and say, "I don't like the science, and therefore I'm going to ignore it", oh my God, I don't know what country that is. What country is that? But what I do know is the country that comes out of that will be sliding backwards, not walking or running forwards.
CURWOOD: We've pretty much stopped space exploration in a big way. We’re doing stuff at the margins, but your book, "Astrophysics for People in a Hurry," really looks at creation of the universe and the basics of physics. It raises lots of questions that would really demand that we look further away from our planet, and it's been a while since we devoted resources to that, a lot of resources.

An artistic rendering of Kepler-452b, the first near-Earth size exoplanet to be discovered. (Photo: NASA/Ames/JPL-Caltech/T. Pyle)
TYSON: Well, yeah, so we are sending space probes, and never at the rate that we always want because we're sort of greedy astrophysicists. But we have probe around Saturn right now. This is last year. There is a probe if you remember that passed Pluto and found another target in the outer solar system. So, it's not like we're not out there. What our dreams were for so many of us was that we would have colonies on Mars, and there would be a human presence. And what has happened over the years is, with the miniaturization of electronics and the development of robotics, a lot of the scientific discoveries you might make by sending a human are done better by sending a robot. So, I get that. Fine.
But the flipside of that is nobody's ever held a ticker tape parade for a robot. Nobody's ever asked a robot to write their life story about what it was for being in space. There's something cultural, something very human, about what it is to touch someone who has been on the frontier of exploration and discovery.
So, you're right, we're not quite there and I'm disappointed. So, something else needs to happen to infuse this kind of dream state in the public, and by the way, because you have very well-read smart listeners to your show, surely a subset of them are saying, “We've got problems on Earth. Why are we spending money in space?” Surely, there's a subset who are thinking that, I can feel it.
CURWOOD: I have to agree.
TYSON: You're out there. I know you're out there. So, here's the point. Consider that Mars once had liquid running water, and it's bone-dry today on its surface. Something bad happened on Mars. We don't know what yet. Mars is to our right. Venus to our left is 900 degrees Fahrenheit. It has a runaway greenhouse effect. Venus is almost exactly the same size and gravity as Earth is. In fact, we called it our sister planet until we got close and said, “Oh, you're 900 degrees. There's nothing sister about you”. So, something bad happened on those planets. I want to know what because I don't want that to happen to Earth. And if you're going to say, “Let's only study Earth and solve our problems here, then look outside”, that's like saying, “We're all huddled in a cave”. And I say, “Gee, I wonder what is on the other side of that mountain”. “No, we can’t look on the other side, we've got to solve the cave problems first. Don't leave the cave”. You realize that's what it looks like to anyone who's in the business of exploring when you say, “Do not explore”.
CURWOOD: There might be one way I could summarize your book, "Astrophysics for People in a Hurry," that, a remark you made a while back, that in many respects, that it's all the same.
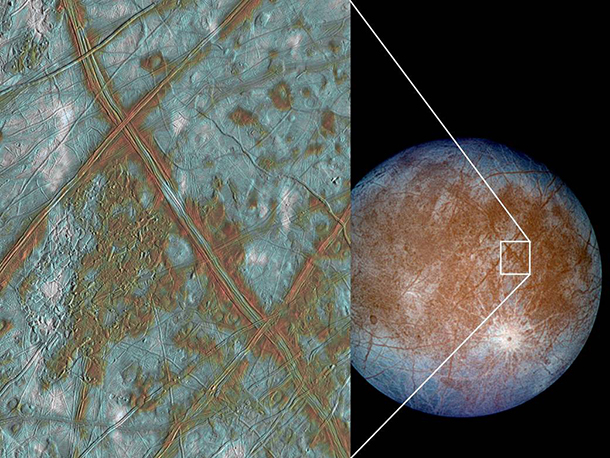
Jupiter's moon Europa has a crust that’s blocks of solid ice, but likely contains liquid water beneath – which means that it could contain microbial life. (Photo: NASA/JPL/University of Arizona)
TYSON: Yeah. One of the most profound discoveries of science is that the laws of nature that we measure here on Earth are the same that exist on the moon, in the sun, across the galaxy, across the universe itself. And that is a remarkable fact when you think about it because here we are on this Earth, this speck called Earth. There's not…Is it written in the sky that says Earth must be the same as everywhere else? If it were not, then science would be a very local affair. Here we would develop all our science just to apply to Earth. If you go to another planet, you’d have to like reinvent other kinds of science and other kinds of laws of physics and chemistry and biology, just to account for what's there. That would be really inefficient but we would do it if we had to. The fact that what we discover here on Earth applies everywhere means we don't have to. I look at the signature of atoms and molecules on Earth, and I find it using spectra, and I find those very same signatures in stars halfway across the universe.
There's not going to be some other planet where Newton's laws somehow don't work. We've measured them to work everywhere, so that sameness, not only in the laws of physics but in the ingredients of the universe, the very atoms that comprise your body, came from a star that gave it's life billions of years ago. We are connected in a fundamental way, and that connectivity for me makes the universe bearable in its infinitude. So many people say, “I feel small in the scale of the universe”. Fine, we are small. Get over it. But because we're the same as the universe, it gives us a deep sense of belonging and participation in this great, unfolding, cosmic story. That's how I and any of my astrophysics colleagues think about this universe.
Neil fuses pop culture and science to connect with his audience.
Today's @Bengals winning OT field goal was likely enabled by a 1/3-in deflection to the right, caused by Earth’s Rotation.
— Neil deGrasse Tyson (@neiltyson) October 11, 2015
CURWOOD: Neil directs the Hayden Planetarium at the American Museum of Natural History in New York. Neil, thanks so much for taking the time today.
TYSON: Yeah, thanks for having me. Always good to be on your show. You know, maybe one day, you can change the name of your show to be Living on Mars.
CURWOOD: [LAUGHS]
Related links:
- Astrophysics for People in a Hurry
- NASA is on a “Journey to Mars”, planning to send humans to Mars in the 2030s
- Neil hosts the radio program Star Talk
- Seeker: “How Mars Went From Warm and Wet to Cold and Dry”
[MUSIC: James Taylor, “Paper Moon” on October Road, composed by Harold Arlen/Yip Harburg/Billy Rose, Sony]
Sounds of Space: The Songs Of Uranus And Neptune
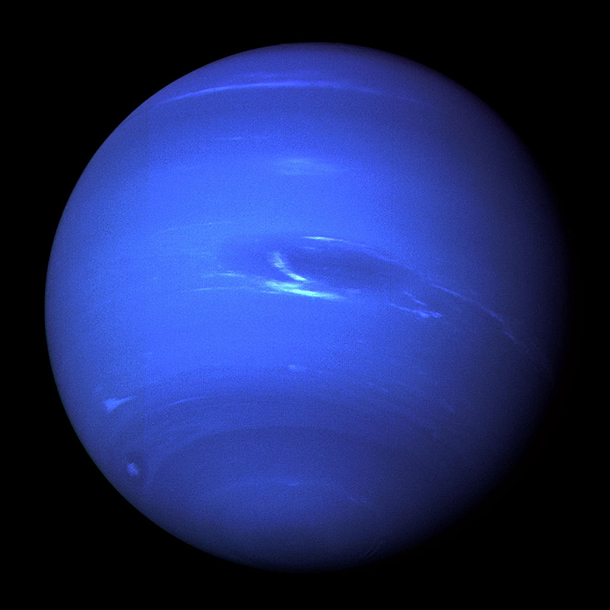
The farthest planet from the Sun, Neptune was named for the Roman god of the sea. (Photo: NASA/JPL)
CURWOOD: We leave you this week listening to the outer reaches of the Solar System.
[SOUNDS OF URANUS]
CURWOOD: Uranus is the seventh planet out from the sun, and NASA has transformed its electromagnetic vibrations into frequencies we can hear.
[URANUS CONTINUES]
CURWOOD: Surrounding Uranus are thirteen rings of dust and debris, and they resonate like a singing bowl.
[RINGING OF RINGS OF URANUS]
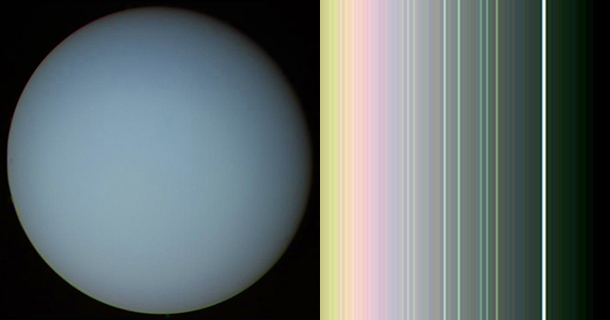
Uranus, and a false-color image of the planet’s rings (which are nearly invisible). (Photo: NASA/JPL)
CURWOOD: Heading further out, you come to Neptune, named for the Roman god of the Sea,
And fittingly, its vibrations evoke waves washing on a shore.
[WHOOSHING OF NEPTUNE]
CURWOOD: There’s more on NASA’S “Symphonies of the Planets” CD.
[NEPTUNE SOUNDS CONTINUE]
Related links:
- About the Voyager mission
- Yes, Uranus has rings!
- The complete Symphonies of the Planets
- More sounds of the solar system from NASA
[MUSIC: Louis Armstrong, “Lovely Weather” (reissued) on Louis Armstrong – Jazz Collection, Delta Records]
CURWOOD: Living on Earth is produced by the World Media Foundation. Our crew includes Naomi Arenberg, Bobby Bascomb, Savannah Christiansen, Jenni Doering, Noble Ingram, Jaime Kaiser, Don Lyman, Alex Metzger, Helen Palmer, Adelaide Chen, and Jolanda Omari. Tom Tiger engineered our show, with help from Jeff Wade and Jake Rego. Alison Lirish Dean composed our themes. You can find us anytime at LOE.org - and like us, please, on our Facebook page - it’s PRI’s Living on Earth. And we tweet from @LivingonEarth. I'm Steve Curwood. Thanks for listening.
ANNOUNCER1: Funding for Living on Earth comes you, our listeners, and from the University of Massachusetts, Boston, in association with its School for the Environment, developing the next generation of environmental leaders. And from the Grantham Foundation for the protection of the environment, supporting strategic communications and collaboration in solving the world’s most pressing environmental problems. Support also comes from the Energy Foundation, serving the public interest by helping to build a strong, clean, energy economy, from Carl and Judy Ferenbach of Boston, Massachusetts and from SolarCity, America’s solar power provider. SolarCity is dedicated to revolutionizing the way energy is delivered by giving customers a renewable alternative to fossil fuels. Information at 888-997-1703. That’s 888-997-1703.
ANNOUNCER2: PRI. Public Radio International.
Living on Earth wants to hear from you!
Living on Earth
62 Calef Highway, Suite 212
Lee, NH 03861
Telephone: 617-287-4121
E-mail: comments@loe.org
Newsletter [Click here]
Donate to Living on Earth!
Living on Earth is an independent media program and relies entirely on contributions from listeners and institutions supporting public service. Please donate now to preserve an independent environmental voice.
NewsletterLiving on Earth offers a weekly delivery of the show's rundown to your mailbox. Sign up for our newsletter today!
 Sailors For The Sea: Be the change you want to sea.
Sailors For The Sea: Be the change you want to sea.
 The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
The Grantham Foundation for the Protection of the Environment: Committed to protecting and improving the health of the global environment.
 Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
Contribute to Living on Earth and receive, as our gift to you, an archival print of one of Mark Seth Lender's extraordinary wildlife photographs. Follow the link to see Mark's current collection of photographs.
 Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth
Buy a signed copy of Mark Seth Lender's book Smeagull the Seagull & support Living on Earth